Lid restraint evokes two types of motor adaptation
- PMID: 11784804
- PMCID: PMC6758672
- DOI: 10.1523/JNEUROSCI.22-02-00569.2002
Lid restraint evokes two types of motor adaptation
Abstract
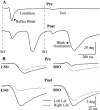
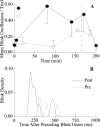
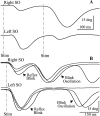
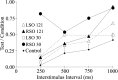
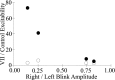
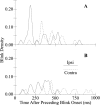
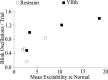
Unilateral reduction in eyelid motility produced two modes of blink adaptation in humans. The first adaptive modification affected both eyelids. Stimulation of the supraorbital branch of the trigeminal nerve (SO) ipsilateral to the upper eyelid with reduced motility evoked bilateral, hyperexcitable reflex blinks, whereas contralateral SO stimulation elicited normally excitable blinks bilaterally. The probability of blink oscillations evoked by stimulation of the ipsilateral SO also increased with a reduction in lid motility. The increased probability of blink oscillations correlated with the enhanced trigeminal reflex blink excitability. Thus, the trigeminal complex ipsilateral to the restrained eyelid coordinated an increase in excitability and blink oscillations independent of the eyelid experiencing reduced motility. The second type of modification appeared only in the eyelid experiencing reduced motility. When tested immediately after removing lid restraint, blink amplitude increased in this eyelid relative to the normal eyelid regardless of the stimulated SO. A patient with seventh nerve palsy exhibited the same two patterns of blink adaptation. These results were consistent with two forms of adaptation, presumably because unilateral lid restraint produced two error signals. The corneal irritation caused by reduced blink amplitude generated abnormal corneal inputs. The difference between proprioceptive feedback from the blink and expected blink magnitude signaled an error in blink amplitude. The corneal irritation appeared to drive an adaptive process organized through the trigeminal complex, whereas the proprioceptive error signal drove an adaptive process involving just the motoneurons controlling the restrained eyelid.
Figures

Similar articles
-
The distribution of primary afferent terminals from the eyelids of macaque monkeys.Exp Brain Res. 1998 Dec;123(4):368-81. doi: 10.1007/s002210050582. Exp Brain Res. 1998. PMID: 9870597
-
Asymmetry of blinking.Invest Ophthalmol Vis Sci. 2006 Jan;47(1):195-201. doi: 10.1167/iovs.04-1279. Invest Ophthalmol Vis Sci. 2006. PMID: 16384962 Free PMC article.
-
Role of proprioception in the control of lid position during reflex and conditioned blink responses in the alert behaving cat.Neuroscience. 1999;90(4):1515-28. doi: 10.1016/s0306-4522(98)00539-9. Neuroscience. 1999. PMID: 10338317
-
Eyelid movements in health and disease. The supranuclear impairment of the palpebral motility.Neurophysiol Clin. 2004 Feb;34(1):3-15. doi: 10.1016/j.neucli.2004.01.002. Neurophysiol Clin. 2004. PMID: 15030796 Review.
-
Spontaneous, Voluntary, and Reflex Blinking in Clinical Practice.J Clin Neurophysiol. 2019 Nov;36(6):415-421. doi: 10.1097/WNP.0000000000000561. J Clin Neurophysiol. 2019. PMID: 31688324 Review.
Cited by
-
Characterizing the spontaneous blink generator: an animal model.J Neurosci. 2011 Aug 3;31(31):11256-67. doi: 10.1523/JNEUROSCI.6218-10.2011. J Neurosci. 2011. PMID: 21813686 Free PMC article.
-
Trigeminal high-frequency stimulation produces short- and long-term modification of reflex blink gain.J Neurophysiol. 2014 Feb;111(4):888-95. doi: 10.1152/jn.00667.2013. Epub 2013 Nov 27. J Neurophysiol. 2014. PMID: 24285868 Free PMC article.
-
Macaque monkey trigeminal blink reflex circuits targeting levator palpebrae superioris motoneurons.J Comp Neurol. 2021 Oct;529(14):3389-3409. doi: 10.1002/cne.25198. Epub 2021 Jun 11. J Comp Neurol. 2021. PMID: 34101199 Free PMC article.
-
Impact of early eyelid weight placement on the development of synkinesis and recovery in patients with idiopathic facial paralysis.World J Otorhinolaryngol Head Neck Surg. 2020 Jun 12;7(4):270-274. doi: 10.1016/j.wjorl.2020.05.005. eCollection 2021 Oct. World J Otorhinolaryngol Head Neck Surg. 2020. PMID: 34632338 Free PMC article.
-
Inactivation of cerebellar output axons impairs acquisition of conditioned eyeblinks.Brain Res. 2006 Nov 29;1122(1):143-53. doi: 10.1016/j.brainres.2006.08.127. Epub 2006 Oct 24. Brain Res. 2006. PMID: 17067561 Free PMC article.
References
-
- Baker RS, Sun WS, Hasan SA, Rouholiman BR, Chuke JC, Cowen DE, Porter JD. Maladaptive neural compensatory mechanisms in Bell's palsy-induced blepharospasm. Neurology. 1997;49:223–229. - PubMed
-
- Basso MA, Strecker RE, Evinger C. Midbrain 6-hydroxydopamine lesions modulate blink reflex excitability. Exp Brain Res. 1993;94:88–96. - PubMed
-
- Bracha V, Bloedel JR. The multiple-pathway model of circuits subserving the classical conditioning of withdrawal reflexes. In: Bloedel JR, Ebner TJ, Wise SP, editors. The acquisition of motor behaviors in vertebrates. MIT; Cambridge, MA: 1996. pp. 175–204.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
