Direct activation of mitogen-activated protein kinase kinase kinase MEKK1 by the Ste20p homologue GCK and the adapter protein TRAF2
- PMID: 11784851
- PMCID: PMC133545
- DOI: 10.1128/MCB.22.3.737-749.2002
Direct activation of mitogen-activated protein kinase kinase kinase MEKK1 by the Ste20p homologue GCK and the adapter protein TRAF2
Abstract
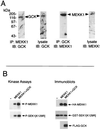
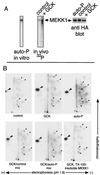
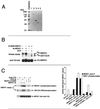
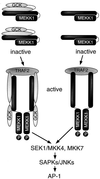
Mitogen-activated protein kinase (MAPK) pathways coordinate critical cellular responses to mitogens, stresses, and developmental cues. The coupling of MAPK kinase kinase (MAP3K) --> MAPK kinase (MEK) --> MAPK core pathways to cell surface receptors remains poorly understood. Recombinant forms of MAP3K MEK kinase 1 (MEKK1) interact in vivo and in vitro with the STE20 protein homologue germinal center kinase (GCK), and both GCK and MEKK1 associate in vivo with the adapter protein tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2). These interactions may couple TNF receptors to the SAPK/JNK family of MAPKs; however, a molecular mechanism by which these proteins might collaborate to recruit the SAPKs/JNKs has remained elusive. Here we show that endogenous GCK and MEKK1 associate in vivo. In addition, we have developed an in vitro assay system with which we demonstrate that purified, active GCK and TRAF2 activate MEKK1. The RING domain of TRAF2 is necessary for optimal in vitro activation of MEKK1, but the kinase domain of GCK is not. Autophosphorylation within the MEKK1 kinase domain activation loop is required for activation. Forced oligomerization also activates MEKK1, and GCK elicits enhanced oligomerization of coexpressed MEKK1 in vivo. These results represent the first activation of MEKK1 in vitro using purified proteins and suggest a mechanism for MEKK1 activation involving induced oligomerization and consequent autophosphorylation mediated by upstream proteins.
Figures
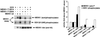
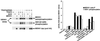




References
-
- Arch, R. H., R. W. Gedrich, and C. B. Thompson. 1998. Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev. 12:2821–2830. - PubMed
-
- Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110–152. - PubMed
-
- Dickens, M., J. S. Rogers, J. Cavanagh, A. Raitano, Z. Xia, J. R. Halpern, M. E. Greenberg, C. L. Sawyers, and R. J. Davis. 1997. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277:693–696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
