Hsp27 as a negative regulator of cytochrome C release
- PMID: 11784858
- PMCID: PMC133538
- DOI: 10.1128/MCB.22.3.816-834.2002
Hsp27 as a negative regulator of cytochrome C release
Abstract
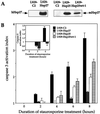
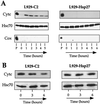
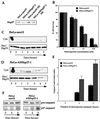
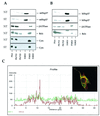
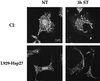
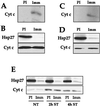
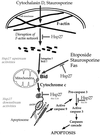
We previously showed that Hsp27 protects against apoptosis through its interaction with cytosolic cytochrome c. We have revisited this protective activity in murine cell lines expressing different levels of Hsp27. We report that Hsp27 also interferes, in a manner dependent on level of expression, with the release of cytochrome c from mitochondria. Moreover, a decreased level of endogenous Hsp27, which sensitized HeLa cells to apoptosis, reduced the delay required for cytochrome c release and procaspase 3 activation. The molecular mechanism regulating this function of Hsp27 is unknown. In our cell systems, Hsp27 is mainly cytosolic and only a small fraction of this protein colocalized with mitochondria. Moreover, we show that only a very small fraction of cytochrome c interacts with Hsp27, hence excluding a role of this interaction in the retention of cytochrome c in mitochondria. We also report that Bid intracellular relocalization was altered by changes in Hsp27 level of expression, suggesting that Hsp27 interferes with apoptotic signals upstream of mitochondria. We therefore investigated if the ability of Hsp27 to act as an expression-dependent modulator of F-actin microfilaments integrity was linked to the retention of cytochrome c in mitochondria. We show here that the F-actin depolymerizing agent cytochalasin D rapidly induced the release of cytochrome c from mitochondria and caspase activation. This phenomenon was delayed in cells pretreated with the F-actin stabilizer phalloidin and in cells expressing a high level of Hsp27. This suggests the existence of an apoptotic signaling pathway linking cytoskeleton damages to mitochondria. This pathway, which induces Bid intracellular redistribution, is negatively regulated by the ability of Hsp27 to protect F-actin network integrity. However, this upstream pathway is probably not the only one to be regulated by Hsp27 since, in staurosporine-treated cells, phalloidin only partially inhibited cytochrome c release and caspase activation. Moreover, in etoposide-treated cells, Hsp27 still delayed the release of cytochrome c from mitochondria and Bid intracellular redistribution in conditions where F-actin was not altered.
Figures







Similar articles
-
Hsp27 negatively regulates cell death by interacting with cytochrome c.Nat Cell Biol. 2000 Sep;2(9):645-52. doi: 10.1038/35023595. Nat Cell Biol. 2000. PMID: 10980706
-
The role of Apaf-1, caspase-9, and bid proteins in etoposide- or paclitaxel-induced mitochondrial events during apoptosis.Cancer Res. 2000 Mar 15;60(6):1645-53. Cancer Res. 2000. PMID: 10749135
-
Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release.Cell Death Differ. 2000 Jun;7(6):556-65. doi: 10.1038/sj.cdd.4400689. Cell Death Differ. 2000. PMID: 10822279
-
The roles of Bid.Apoptosis. 2002 Oct;7(5):433-40. doi: 10.1023/a:1020035124855. Apoptosis. 2002. PMID: 12207176 Review.
-
Heat shock proteins: essential proteins for apoptosis regulation.J Cell Mol Med. 2008 Jun;12(3):743-61. doi: 10.1111/j.1582-4934.2008.00273.x. Epub 2008 Feb 8. J Cell Mol Med. 2008. PMID: 18266962 Free PMC article. Review.
Cited by
-
Molecular Mechanisms and the Interplay of Important Chronic Obstructive Pulmonary Disease Biomarkers Reveals Novel Therapeutic Targets.ACS Omega. 2023 Nov 20;8(49):46376-46389. doi: 10.1021/acsomega.3c07480. eCollection 2023 Dec 12. ACS Omega. 2023. PMID: 38107961 Free PMC article. Review.
-
Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells.Cell Stress Chaperones. 2005 Autumn;10(3):252-62. doi: 10.1379/csc-124r.1. Cell Stress Chaperones. 2005. PMID: 16184770 Free PMC article.
-
Inhibition of apoptosis by p26: implications for small heat shock protein function during Artemia development.Cell Stress Chaperones. 2006 Spring;11(1):71-80. doi: 10.1379/csc-154r.1. Cell Stress Chaperones. 2006. PMID: 16572731 Free PMC article.
-
Heat shock protein 27: its potential role in vascular disease.Int J Exp Pathol. 2006 Aug;87(4):253-74. doi: 10.1111/j.1365-2613.2006.00484.x. Int J Exp Pathol. 2006. PMID: 16875491 Free PMC article. Review.
-
HSP25 down-regulation enhanced p53 acetylation by dissociation of SIRT1 from p53 in doxorubicin-induced H9c2 cell apoptosis.Cell Stress Chaperones. 2016 Mar;21(2):251-60. doi: 10.1007/s12192-015-0655-3. Epub 2015 Oct 29. Cell Stress Chaperones. 2016. PMID: 26515559 Free PMC article.
References
-
- Arrigo, A.-P., and J. Landry. 1994. Expression and function of the low-molecular-weight heat shock proteins, p.335–373. In R. I. Morimoto, A. Tissieres, and C. Georgopoulos (ed.), The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
-
- Arrigo, A. P. 1998. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol. Chem. 379:19–26. - PubMed
-
- Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the apaf-1 apoptosome. Nat. Cell Biol. 2:469–475. - PubMed
-
- Benndorf, R., K. Hayess, S. Ryazantsev, M. Wieske, J. Behlke, and G. Lutsch. 1994. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J. Biol. Chem. 269:20780–20784. - PubMed
-
- Beresford, P. J., M. Jaju, R. S. Friedman, M. J. Yoon, and J. Lieberman. 1998. A role for heat shock protein 27 in CTL-mediated cell death. J. Immunol. 161:161–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
