Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice
- PMID: 11784860
- PMCID: PMC133561
- DOI: 10.1128/MCB.22.3.849-855.2002
Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice
Abstract
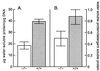
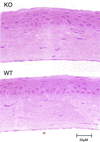
We have constructed an ALDH3a1 null mouse to investigate the role of this enzyme that comprises nearly one-half of the total water-soluble protein in the mouse corneal epithelium. ALDH3a1-deficient mice are viable and fertile, have a corneal epithelium with a water-soluble protein content approximately half that of wild-type mice, and contain no ALDH3a1 as determined by zymograms and immunoblots. Despite the loss of protein content and ALDH3a1 activity, the ALDH3a1(-/-) mouse corneas appear indistinguishable from wild-type corneas when examined by histological analysis and electron microscopy and are transparent as determined by light and slit lamp microscopy. There is no evidence for a compensating protein or enzyme. Even though the function of ALDH3a1 in the mouse cornea remains unknown, our data indicate that its enzymatic activity is unnecessary for corneal clarity and maintenance, at least under laboratory conditions.
Figures





Similar articles
-
Alkali burn causes aldehyde dehydrogenase 3A1 (ALDH3A1) decrease in mouse cornea.Mol Vis. 2004 Nov 8;10:845-50. Mol Vis. 2004. PMID: 15547490
-
Gene expression of the mouse corneal crystallin Aldh3a1: activation by Pax6, Oct1, and p300.Invest Ophthalmol Vis Sci. 2008 May;49(5):1814-26. doi: 10.1167/iovs.07-1057. Invest Ophthalmol Vis Sci. 2008. PMID: 18436815
-
Corneal haze phenotype in Aldh3a1-null mice: In vivo confocal microscopy and tissue imaging mass spectrometry.Chem Biol Interact. 2017 Oct 1;276:9-14. doi: 10.1016/j.cbi.2016.12.017. Epub 2016 Dec 27. Chem Biol Interact. 2017. PMID: 28038895
-
ALDH3A1: a corneal crystallin with diverse functions.Exp Eye Res. 2007 Jan;84(1):3-12. doi: 10.1016/j.exer.2006.04.010. Epub 2006 Jun 21. Exp Eye Res. 2007. PMID: 16797007 Review.
-
Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the "refracton" hypothesis.Cornea. 2001 Nov;20(8):853-8. doi: 10.1097/00003226-200111000-00015. Cornea. 2001. PMID: 11685065 Review.
Cited by
-
Composition, structure and function of the corneal stroma.Exp Eye Res. 2020 Sep;198:108137. doi: 10.1016/j.exer.2020.108137. Epub 2020 Jul 11. Exp Eye Res. 2020. PMID: 32663498 Free PMC article. Review.
-
Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(-/-)/Aldh1a1(-/-) knock-out mice.J Biol Chem. 2007 Aug 31;282(35):25668-76. doi: 10.1074/jbc.M702076200. Epub 2007 Jun 13. J Biol Chem. 2007. PMID: 17567582 Free PMC article.
-
Corneal crystallins and the development of cellular transparency.Semin Cell Dev Biol. 2008 Apr;19(2):82-93. doi: 10.1016/j.semcdb.2007.09.015. Epub 2007 Oct 2. Semin Cell Dev Biol. 2008. PMID: 17997336 Free PMC article. Review.
-
Preferential transcription of rabbit Aldh1a1 in the cornea: implication of hypoxia-related pathways.Mol Cell Biol. 2004 Feb;24(3):1324-40. doi: 10.1128/MCB.24.3.1324-1340.2004. Mol Cell Biol. 2004. PMID: 14729976 Free PMC article.
-
Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily.Expert Opin Drug Metab Toxicol. 2008 Jun;4(6):697-720. doi: 10.1517/17425255.4.6.697. Expert Opin Drug Metab Toxicol. 2008. PMID: 18611112 Free PMC article. Review.
References
-
- Abedinia, M., T. Pain, E. M. Algar, and R. S. Holmes. 1990. Bovine corneal aldehyde dehydrogenase: the major soluble corneal protein with a possible dual protective role for the eye. Exp. Eye Res. 51:419–426. - PubMed
-
- Alexander, R. J., B. Silverman, and W. L. Henley. 1981. Isolation and characterization of BCP 54, the major soluble protein of bovine cornea. Exp. Eye Res. 32:205–216. - PubMed
-
- Algar, E. M., M. Abedinia, J. L. VandeBerg, and R. S. Holmes. 1991. Purification and properties of baboon corneal aldehyde dehydrogenase: proposed UVR protective role. Adv. Exp. Med. Biol. 284:53–60. - PubMed
-
- Blaheta, R. A., B. Kronenberger, D. Woitaschek, S. Weber, M. Scholz, H. Schuldes, A. Encke, and B. H. Markus. 1998. Development of an ultrasensitive in vitro assay to monitor growth of primary cell cultures with reduced mitotic activity. J. Immunol. Methods 211:159–169. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
