Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases
- PMID: 11784863
- PMCID: PMC133550
- DOI: 10.1128/MCB.22.3.874-885.2002
Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases
Abstract
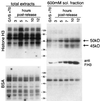
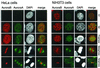
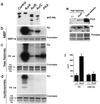
Phosphorylation at a highly conserved serine residue (Ser-10) in the histone H3 tail is considered to be a crucial event for the onset of mitosis. This modification appears early in the G(2) phase within pericentromeric heterochromatin and spreads in an ordered fashion coincident with mitotic chromosome condensation. Mutation of Ser-10 is essential in Tetrahymena, since it results in abnormal chromosome segregation and extensive chromosome loss during mitosis and meiosis, establishing a strong link between signaling and chromosome dynamics. Although mitotic H3 phosphorylation has been long recognized, the transduction routes and the identity of the protein kinases involved have been elusive. Here we show that the expression of Aurora-A and Aurora-B, two kinases of the Aurora/AIK family, is tightly coordinated with H3 phosphorylation during the G(2)/M transition. During the G(2) phase, the Aurora-A kinase is coexpressed while the Aurora-B kinase colocalizes with phosphorylated histone H3. At prophase and metaphase, Aurora-A is highly localized in the centrosomic region and in the spindle poles while Aurora-B is present in the centromeric region concurrent with H3 phosphorylation, to then translocate by cytokinesis to the midbody region. Both Aurora-A and Aurora-B proteins physically interact with the H3 tail and efficiently phosphorylate Ser10 both in vitro and in vivo, even if Aurora-A appears to be a better H3 kinase than Aurora-B. Since Aurora-A and Aurora-B are known to be overexpressed in a variety of human cancers, our findings provide an attractive link between cell transformation, chromatin modifications and a specific kinase system.
Figures





References
-
- Ajiro, K., K. Yoda, K. Utsumi, and Y. Nishikawa. 1996. Alteration of cell cycle-dependent histone phosphorylations by okadaic acid. Induction of mitosis-specific H3 phosphorylation and chromatin condensation in mammalian interphase cells. J. Biol. Chem. 271:13197–13201. - PubMed
-
- Bischoff, J. R., L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanagan, F. Clairvoyant, C. Ginther, C. S. Chan, M. Novotny, D. J. Slamon, and G. D. Plowman. 1998. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17:3052–3065. - PMC - PubMed
-
- Bischoff, J. R., and G. D. Plowman. 1999. The Aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol. 9:454–459. - PubMed
-
- Bradbury, E. M. 1992. Reversible histone modifications and the chromosome cell cycle. Bioessays 14:9–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
