Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation
- PMID: 11786397
- PMCID: PMC1867115
- DOI: 10.1016/S0002-9440(10)64347-7
Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation
Abstract
Using the National Center for Biotechnology Information Serial Analysis of Gene Expression database, we found that S100A4, a calcium-binding protein previously implicated in metastasis, was expressed in five of seven pancreatic carcinoma libraries but not in the two normal pancreatic duct libraries. We confirmed the overexpression of S100A4 using reverse transcriptase-polymerase chain reaction, which demonstrated that 18 of 19 (95%) pancreatic carcinoma cell lines expressed S100A4. Using immunohistochemistry, we found that 57 of 61 invasive pancreatic carcinomas (93%), 3 of 18 high-grade pancreatic intraepithelial neoplasia lesions (17%), and 0 of the 69 low-grade pancreatic intraepithelial neoplasia lesions expressed S100A4 protein, whereas normal pancreatic tissue and tissue affected by chronic pancreatitis did not label. Expression of S100A4 was associated with poor differentiation of the pancreatic adenocarcinomas (P = 0.001). We found that three CpG sites in the first intron of the S100A4 gene were approximately 90% methylated in microdissected normal pancreatic duct cells using bisulfite-modified sequencing and in two cell lines and three primary pancreatic carcinomas with a reduced or absent expression of S100A4. In contrast, these CpGs were 100% hypomethylated in 11 of 12 pancreatic cancer cell lines by methylation-specific polymerase chain reaction. The association between the expression of S100A4 and hypomethylation of the first intron of S100A4 was statistically significant (P = 0.002). These data suggest that the majority of pancreatic carcinomas undergo selection for hypomethylation and overexpression of S100A4. Because most pancreatic carcinomas express S100A4, it may be a useful target for early detection strategies.
Figures

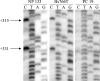
Comment in
-
Protein S100A4: too long overlooked by pathologists?Am J Pathol. 2002 Jan;160(1):7-13. doi: 10.1016/S0002-9440(10)64342-8. Am J Pathol. 2002. PMID: 11786392 Free PMC article. No abstract available.
References
-
- Schafer BW, Heizmann CW: The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci 1996, 21:134-140 - PubMed
-
- De Vouge MW, Mukherjee BB: Transformation of normal rat kidney cells by v-K-ras enhances expression of transin 2 and an S-100-related calcium-binding protein. Oncogene 1992, 7:109-119 - PubMed
-
- Goto K, Endo H, Fujiyoshi T: Cloning of the sequences expressed abundantly in established cell lines: identification of a cDNA clone highly homologous to S-100, a calcium binding protein. J Biochem 1988, 103:48-53 - PubMed
-
- Davies BR, Davies MP, Gibbs FE, Barraclough R, Rudland PS: Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for p9Ka, a rat calcium-binding protein, but not with the oncogene EJ-ras-1. Oncogene 1993, 8:999-1008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

