CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils
- PMID: 11786404
- PMCID: PMC1867121
- DOI: 10.1016/s0002-9440(10)64354-4
CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils
Abstract
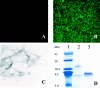
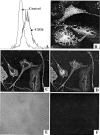
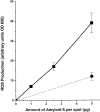
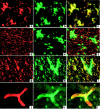
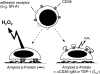
A pathological hallmark of Alzheimer's disease is the senile plaque, composed of beta-amyloid fibrils, microglia, astrocytes, and dystrophic neurites. We reported previously that class A scavenger receptors mediate adhesion of microglia and macrophages to beta-amyloid fibrils and oxidized low-density lipoprotein (oxLDL)-coated surfaces. We also showed that CD36, a class B scavenger receptor and an oxLDL receptor, promotes H(2)O(2) secretion by macrophages adherent to oxLDL-coated surfaces. Whether CD36 is expressed on microglia, and whether it plays a role in secretion of H(2)O(2) by microglia interacting with fibrillar beta-amyloid is not known. Using fluorescence-activated cell sorting analysis and immunohistochemistry, we found that CD36 is expressed on human fetal microglia, and N9-immortalized mouse microglia. We also found that CD36 is expressed on microglia and on vascular endothelial cells in the brains of Alzheimer's disease patients. Bowes human melanoma cells, which normally do not express CD36, gained the ability to specifically bind to surfaces coated with fibrillar beta-amyloid when transfected with a cDNA encoding human CD36, suggesting that CD36 is a receptor for fibrillar beta-amyloid. Furthermore, two different monoclonal antibodies to CD36 inhibited H(2)O(2) production by N9 microglia and human macrophages adherent to fibrillar beta-amyloid by approximately 50%. Our data identify a role for CD36 in fibrillar beta-amyloid-induced H(2)O(2) production by microglia, and imply that CD36 can mediate binding to fibrillar beta-amyloid. We propose that similar to their role in the interaction of macrophages with oxLDL, class A scavenger receptors and CD36 play complimentary roles in the interactions of microglia with fibrillar beta-amyloid.
Figures
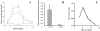
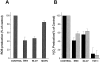
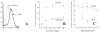
References
-
- Selkoe DJ: The origins of Alzheimer disease: a is for amyloid. JAMA 2000, 283:1615-1617 - PubMed
-
- Wisniewski HM, Robe A, Zigman W, Silverman W: Neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol 1989, 48:606-609 - PubMed
-
- El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC: Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol Aging 1998, 19:S81-S84 - PubMed
-
- Akiyama H: Inflammatory response in Alzheimer’s disease. Tohoku J Exp Med 1994, 174:295-303 - PubMed
-
- Meda L, Cassatella MA, Szendrei GI, Otvos Jr L, Baron P, Villalba M, Ferrari D, Rossi F: Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 1995, 374:647–650 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

