The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis
- PMID: 11786413
- PMCID: PMC1867136
- DOI: 10.1016/s0002-9440(10)64363-5
The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis
Abstract
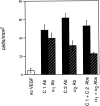
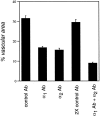
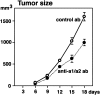
Angiogenesis is a complex process, involving functional cooperativity between cytokines and endothelial cell (EC) surface integrins. In this study, we investigated the mechanisms through which the alpha(1)beta(1) and alpha(2)beta(1) integrins support angiogenesis driven by vascular endothelial growth factor (VEGF). Dermal microvascular EC attachment through either alpha(1)beta(1) or alpha(2)beta(1) supported robust VEGF activation of the Erk1/Erk2 (p44/42) mitogen-activated protein kinase signal transduction pathway that drives EC proliferation. Haptotactic EC migration toward collagen I was dependent on alpha(1)beta(1) and alpha(2)beta(1) as was VEGF-stimulated chemotaxis of ECs in a uniform collagen matrix. Consistent with the functions of alpha(1)beta(1) and alpha(2)beta(1) in supporting signal transduction and EC migration, antibody antagonism of either integrin resulted in potent inhibition of VEGF-driven angiogenesis in mouse skin. Moreover, combined antagonism of alpha(1)beta(1) and alpha(2)beta(1) substantially reduced tumor growth and angiogenesis of human squamous cell carcinoma xenografts. Collectively, these studies identify critical collaborative functions for the alpha(1)beta(1) and alpha(2)beta(1) integrins in supporting VEGF signal transduction, EC migration, and tumor angiogenesis.
Figures




References
-
- Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF: Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 1993, 12:303-324 - PubMed
-
- Ferrara N: The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat 1995, 36:127-137 - PubMed
-
- Ferrara N, Henzel WJ: Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989, 161:851-858 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

