Inhibition of cytoskeletal assembly by cytochalasin B prevents signaling through tyrosine phosphorylation and secretion triggered by collagen but not by thrombin
- PMID: 11786426
- PMCID: PMC1867124
- DOI: 10.1016/S0002-9440(10)64376-3
Inhibition of cytoskeletal assembly by cytochalasin B prevents signaling through tyrosine phosphorylation and secretion triggered by collagen but not by thrombin
Abstract
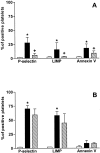
Activation of platelets leads to cytoskeletal assembly that is responsible for platelet motility and internal contraction. We have evaluated the involvement of the cytoskeleton in platelet activation by two strong agonists, collagen and thrombin. Activation was assessed by measuring changes in cytoskeletal assembly, externalization of activation-dependent markers and expression of procoagulant activity, and tyrosine phosphorylation of proteins, in both the absence and the presence of cytochalasin B. Activation of platelets with collagen and thrombin induced morphological changes and increased the expression of CD62P, CD63, glycoprotein IV, and binding of annexin V to platelets. Moreover, both activating agents induced actin polymerization, increased the association of other contractile proteins, and promoted tyrosine phosphorylation of multiple proteins, some of which were associated with the cytoskeleton. The presence of cytochalasin B blocked the previous events when collagen was used as the activating agent, although binding of annexin V still occurred. In contrast, platelet response to thrombin was not completely prevented by the presence of cytochalasin B. Thus, activation by collagen requires a functional cytoskeleton to trigger signaling through tyrosine phosphorylation and secretion. This is not the case for thrombin, which is capable of activating signaling mechanisms in the presence of strong inhibitors of cytoskeletal assembly. Moreover, the expression of a procoagulant surface in platelets still occurs even when platelet motility has been inhibited.
Figures



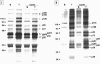
References
-
- Clemetson KJ: Platelet collagen receptors: a new target for inhibition? Haemostasis 1999, 29:16-26 - PubMed
-
- Weiss HJ, Lages B: Evidence for tissue factor-dependent activation of the classic extrinsic coagulation mechanism in blood obtained from bleeding time wounds. Blood 1988, 71:629-635 - PubMed
-
- Wagner WR, Hubbell JA: Local thrombin synthesis and fibrin formation in an in vitro thrombosis model result in platelet recruitment and thrombus stabilization on collagen in heparinized blood. J Lab Clin Med 1990, 116:636-650 - PubMed
-
- Diaz-Ricart M, Estebanell E, Lozano M, Aznar-Salatti J, White JG, Ordinas A, Escolar G: Thrombin facilitates primary platelet adhesion onto vascular surfaces in the absence of plasma adhesive proteins: studies under flow conditions. Haematologica 2000, 85:280-288 - PubMed
-
- White JG: Platelet membrane ultrastructure and its changes during platelet activation. Prog Clin Biol Res 1988, 283:1-32 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

