High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells
- PMID: 11788735
- PMCID: PMC99848
- DOI: 10.1093/nar/30.2.e9
High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells
Abstract
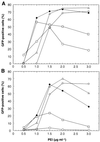
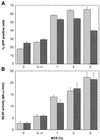
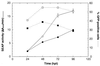
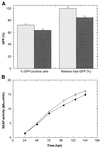
A scalable transfection procedure using polyethylenimine (PEI) is described for the human embryonic kidney 293 cell line grown in suspension. Green fluorescent protein (GFP) and human placental secreted alkaline phosphatase (SEAP) were used as reporter genes to monitor transfection efficiency and productivity. Up to 75% of GFP-positive cells were obtained using linear or branched 25 kDa PEI. The 293 cell line and two genetic variants, either expressing the SV40 large T-antigen (293T) or the Epstein-Barr virus (EBV) EBNA1 protein (293E), were tested for protein expression. The highest expression level was obtained with 293E cells using the EBV oriP-containing plasmid pCEP4. We designed the pTT vector, an oriP-based vector having an improved cytomegalovirus expression cassette. Using this vector, 10- and 3-fold increases in SEAP expression was obtained in 293E cells compared with pcDNA3.1 and pCEP4 vectors, respectively. The presence of serum had a positive effect on gene transfer and expression. Transfection of suspension-growing cells was more efficient with linear PEI and was not affected by the presence of medium conditioned for 24 h. Using the pTT vector, >20 mg/l of purified His-tagged SEAP was recovered from a 3.5 l bioreactor. Intracellular proteins were also produced at levels as high as 50 mg/l, representing up to 20% of total cell proteins.
Figures


References
-
- Cullen B.R. (1987) Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol., 152, 684–704. - PubMed
-
- Blasey H.D., Aubry,J.-P., Mazzei,G. and Bernard,A. (1996) Large scale transient expression with COS cells. Cytotechnology, 18, 183–192. - PubMed
-
- Cachianes G., Ho,C., Weber,R.F., Williams,S.R., Goeddel,D.V. and Leung,D.W. (1993) Epstein–Barr virus-derived vectors for transient and stable expression of recombinant proteins. Biotechniques, 15, 255–259. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

