Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis
- PMID: 11788757
- PMCID: PMC148950
Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis
Abstract
The arbuscular mycorrhizal (AM) symbiosis is responsible for huge fluxes of photosynthetically fixed carbon from plants to the soil. Carbon is transferred from the plant to the fungus as hexose, but the main form of carbon stored by the mycobiont at all stages of its life cycle is triacylglycerol. Previous isotopic labeling experiments showed that the fungus exports this storage lipid from the intraradical mycelium (IRM) to the extraradical mycelium (ERM). Here, in vivo multiphoton microscopy was used to observe the movement of lipid bodies through the fungal colony and to determine their sizes, distribution, and velocities. The distribution of lipid bodies along fungal hyphae suggests that they are progressively consumed as they move toward growing tips. We report the isolation and measurements of expression of an AM fungal expressed sequence tag that encodes a putative acyl-coenzyme A dehydrogenase; its deduced amino acid sequence suggests that it may function in the anabolic flux of carbon from lipid to carbohydrate. Time-lapse image sequences show lipid bodies moving in both directions along hyphae and nuclear magnetic resonance analysis of labeling patterns after supplying 13C-labeled glycerol to either extraradical hyphae or colonized roots shows that there is indeed significant bidirectional translocation between IRM and ERM. We conclude that large amounts of lipid are translocated within the AM fungal colony and that, whereas net movement is from the IRM to the ERM, there is also substantial recirculation throughout the fungus.
Figures
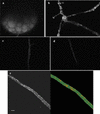




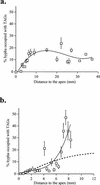
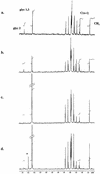

References
-
- Aist JR. Independent nuclear motility and hyphal tip growth. Can J Bot. 1995;73:S122–125.
-
- Åström H, Giovannetti M, Raudaskoski M. Cytoskeletal components in the arbuscular mycorrhizal fungus Glomus mosseae. Mol Plant Microbe Interact. 1994;7:309–312.
-
- Bago B, Azcón-Aguilar C, Goulet A, Piché Y. Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 1998a;139:375–388.
-
- Bago B, Azcón-Aguilar C, Piché Y. Architecture and developmental dynamics of the external mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown under monoxenic conditions. Mycologia. 1998b;90:52–62.
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
