Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence
- PMID: 11790730
- PMCID: PMC139534
- DOI: 10.1128/JB.184.3.621-628.2002
Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence
Abstract
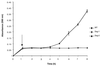
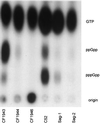
We describe here the identification and characterization of two Listeria monocytogenes (Tn917-LTV3) relA and hpt transposon insertion mutants that were impaired in growth after attachment to a model surface. Both mutants were unable to accumulate (p)ppGpp in response to amino acid starvation, whereas the wild-type strain accumulated (p)ppGpp within 30 min of stress induction. The induction of transcription of the relA gene after adhesion was demonstrated, suggesting that the ability to mount a stringent response and undergo physiological adaptation to nutrient deprivation is essential for the subsequent growth of the adhered bacteria. The absence of (p)ppGpp in the hpt mutant, which is blocked in the purine salvage pathway, is curious and suggests that a functional purine salvage pathway is required for the biosynthesis of (p)ppGpp. Both mutants were avirulent in a murine model of listeriosis, indicating an essential role for the stringent response in the survival and growth of L. monocytogenes in the host. Taken as a whole, this study provides new information on the role of the stringent response and the physiological adaptation of L. monocytogenes for biofilm growth and pathogenesis.
Figures



Similar articles
-
Cloning of rel from Listeria monocytogenes as an osmotolerance involvement gene.Appl Environ Microbiol. 2002 Apr;68(4):1541-7. doi: 10.1128/AEM.68.4.1541-1547.2002. Appl Environ Microbiol. 2002. PMID: 11916666 Free PMC article.
-
A Listeria monocytogenes mutant defective in bacteriophage attachment is attenuated in orally inoculated mice and impaired in enterocyte intracellular growth.Infect Immun. 2008 Sep;76(9):4046-54. doi: 10.1128/IAI.00283-08. Epub 2008 Jun 16. Infect Immun. 2008. PMID: 18559424 Free PMC article.
-
Reduced host cell invasiveness and oxidative stress tolerance in double and triple csp gene family deletion mutants of Listeria monocytogenes.Foodborne Pathog Dis. 2010 Jul;7(7):775-83. doi: 10.1089/fpd.2009.0458. Foodborne Pathog Dis. 2010. PMID: 20184451
-
RelA alone appears essential for (p)ppGpp production when Neisseria gonorrhoeae encounters nutritional stress.FEMS Microbiol Lett. 2005 Jul 1;248(1):1-8. doi: 10.1016/j.femsle.2005.05.014. FEMS Microbiol Lett. 2005. PMID: 15936895
-
The Listeria monocytogenes homolog of the Escherichia coli era gene is involved in adhesion to inert surfaces.Appl Environ Microbiol. 2007 Dec;73(23):7789-92. doi: 10.1128/AEM.01157-07. Epub 2007 Oct 5. Appl Environ Microbiol. 2007. PMID: 17921262 Free PMC article.
Cited by
-
The role of ClpP in protein expression of Streptococcus pneumoniae.Curr Microbiol. 2012 Mar;64(3):294-9. doi: 10.1007/s00284-011-0060-9. Epub 2011 Dec 25. Curr Microbiol. 2012. PMID: 22198546
-
Bacterial cell attachment, the beginning of a biofilm.J Ind Microbiol Biotechnol. 2007 Sep;34(9):577-88. doi: 10.1007/s10295-007-0234-4. Epub 2007 Jul 6. J Ind Microbiol Biotechnol. 2007. PMID: 17619090 Review.
-
Persistence of Intracellular Bacterial Pathogens-With a Focus on the Metabolic Perspective.Front Cell Infect Microbiol. 2021 Jan 14;10:615450. doi: 10.3389/fcimb.2020.615450. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33520740 Free PMC article. Review.
-
ActA promotes Listeria monocytogenes aggregation, intestinal colonization and carriage.PLoS Pathog. 2013 Jan;9(1):e1003131. doi: 10.1371/journal.ppat.1003131. Epub 2013 Jan 31. PLoS Pathog. 2013. PMID: 23382675 Free PMC article.
-
The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis.J Bacteriol. 2009 Apr;191(7):2248-56. doi: 10.1128/JB.01726-08. Epub 2009 Jan 23. J Bacteriol. 2009. PMID: 19168608 Free PMC article.
References
-
- Brown, M. 1996. Studies on the adhesion of Listeria monocytogenes to leaf and model surfaces. Ph.D thesis. University of Manchester, Manchester, United Kingdom.
-
- Cashel, M. 1994. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants, p.341–356. In, C. W. Adolph (ed.), Methods in molecular genetics, vol. 3. Academic Press, Inc., San Diego, Calif.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

