Novel type of ADP-forming acetyl coenzyme A synthetase in hyperthermophilic archaea: heterologous expression and characterization of isoenzymes from the sulfate reducer Archaeoglobus fulgidus and the methanogen Methanococcus jannaschii
- PMID: 11790732
- PMCID: PMC139507
- DOI: 10.1128/JB.184.3.636-644.2002
Novel type of ADP-forming acetyl coenzyme A synthetase in hyperthermophilic archaea: heterologous expression and characterization of isoenzymes from the sulfate reducer Archaeoglobus fulgidus and the methanogen Methanococcus jannaschii
Abstract
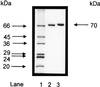
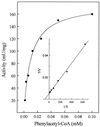
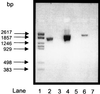
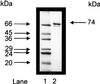
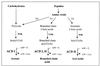
Acetyl coenzyme A (CoA) synthetase (ADP forming) (ACD) represents a novel enzyme of acetate formation and energy conservation (acetyl-CoA + ADP + P(i) right harpoon over left harpoon acetate + ATP + CoA) in Archaea and eukaryotic protists. The only characterized ACD in archaea, two isoenzymes from the hyperthermophile Pyrococcus furiosus, constitute 145-kDa heterotetramers (alpha(2), beta(2)). The coding genes for the alpha and beta subunits are located at different sites in the P. furiosus chromosome. Based on significant sequence similarity of the P. furiosus genes, five open reading frames (ORFs) encoding putative ACD were identified in the genome of the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus and one ORF was identified in the hyperthermophilic methanogen Methanococcus jannaschii. The ORFs constitute fusions of the homologous P. furiosus genes encoding the alpha and beta subunits. Two ORFs, AF1211 and AF1938, of A. fulgidus and ORF MJ0590 of M. jannaschii were cloned and functionally overexpressed in Escherichia coli. The purified recombinant proteins were characterized as distinctive isoenzymes of ACD with different substrate specificities. In contrast to the Pyrococcus ACD, the ACDs of Archaeoglobus and Methanococcus constitute homodimers of about 140 kDa composed of two identical 70-kDa subunits, which represent fusions of the homologous P. furiosus alpha and beta subunits in an alphabeta (AF1211 and MJ0590) or betaalpha (AF1938) orientation. The data indicate that A. fulgidus and M. jannaschii contains a novel type of ADP-forming acetyl-CoA synthetase in Archaea, in which the subunit polypeptides and their coding genes are fused.
Figures




References
-
- Adams, M. W., J. F. Holden, A. L. Menon, G. J. Schut, A. M. Grunden, C. Hou, A. M. Hutchins, F. E. Jenney, C. Kim, K. Ma, G. Pan, R. Roy, R. Sapra, S. V. Story, and M. F. Verhagen. 2001. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183:716–724. - PMC - PubMed
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. - PubMed
-
- Bult, C. J., O. White, G. J. Olsen, L. Zhou, R. D. Fleischmann, G. G. Sutton, J. A. Blake, L. M. Fitzgerald, R. A. Clayton, J. D. Gocayne, A. R. Kerlavage, B. A. Dougherty, J. F. Tomb, M. D. Adams, C. I. Reich, R. Overbeek, E. F. Kirkness, K. G. Weinstock, J. M. Merrick, A. Glodek, J. L. Scott, N. S. M. Geoghagen, and J. C. Venter. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058–1073. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

