High-salinity-induced iron limitation in Bacillus subtilis
- PMID: 11790741
- PMCID: PMC139516
- DOI: 10.1128/JB.184.3.718-727.2002
High-salinity-induced iron limitation in Bacillus subtilis
Abstract
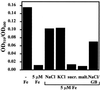
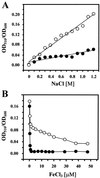
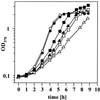
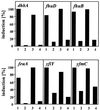
Proteome analysis of Bacillus subtilis cells grown at low and high salinity revealed the induction of 16 protein spots and the repression of 2 protein spots, respectively. Most of these protein spots were identified by mass spectrometry. Four of the 16 high-salinity-induced proteins corresponded to DhbA, DhbB, DhbC, and DhbE, enzymes that are involved in the synthesis of 2,3-dihydroxybenzoate (DHB) and its modification and esterification to the iron siderophore bacillibactin. These proteins are encoded by the dhbACEBF operon, which is negatively controlled by the central iron regulatory protein Fur and is derepressed upon iron limitation. We found that iron limitation and high salinity derepressed dhb expression to a similar extent and that both led to the accumulation of comparable amounts of DHB in the culture supernatant. DHB production increased linearly with the degree of salinity of the growth medium but could still be reduced by an excess of iron. Such an excess of iron also partially reversed the growth defect exhibited by salt-stressed B. subtilis cultures. Taken together, these findings strongly suggest that B. subtilis cells grown at high salinity experience iron limitation. In support of this notion, we found that the expression of several genes and operons encoding putative iron uptake systems was increased upon salt stress. The unexpected finding that high-salinity stress has an iron limitation component might be of special ecophysiological importance for the growth of B. subtilis in natural settings, in which bioavailable iron is usually scarce.
Figures




References
-
- Alice, A. F., and C. Sanchez-Rivas. 1997. DNA supercoiling and osmoresistance in Bacillus subtilis 168. Curr. Microbiol. 35:309–315. - PubMed
-
- Bloom, H., H. Beier, and H. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

