Stimulation of recombinant Ca(v)3.2, T-type, Ca(2+) channel currents by CaMKIIgamma(C)
- PMID: 11790804
- PMCID: PMC2290082
- DOI: 10.1113/jphysiol.2001.012839
Stimulation of recombinant Ca(v)3.2, T-type, Ca(2+) channel currents by CaMKIIgamma(C)
Abstract
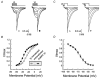
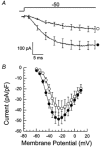
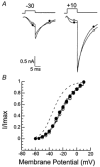
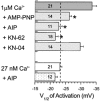
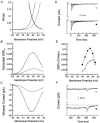
Molecular cloning of low-voltage activated (LVA) T-type calcium channels has enabled the study of their regulation in heterologous expression systems. Here we investigate the regulation of Ca(v)3.2 alpha(1)-subunits (alpha1H) by calcium- and/or calmodulin-dependent protein kinase II (CaMKII). 293 cells stably expressing alpha1H were transiently transfected with CaMKIIgamma(C). Using the whole-cell recording configuration, we observed that elevation of pipette free Ca(2+) (1 microM) in the presence of CaM (2 microM) increases T-type channel activity selectively at negative potentials, evoking an 11 mV hyperpolarizing shift in the half-maximal potential (V(1/2)) for activation. The V(1/2) of channel inactivation is not altered by Ca(2+)/CaM. These effects reproduced modulation observed in adrenal zona glomerulosa cells. The potentiation by Ca(2+)/CaM was dependent on the co-expression of CaMKIIgamma(C) and required Ca(2+)/CaM-dependent kinase activity. Peptide (AIP) and lipophilic (KN-62) protein kinase inhibitors prevented the Ca(2+)/CaM-induced changes in channel gating without altering basal Ca(v)3.2 channel activity (27 nM free Ca(2+)) as did replacing pipette ATP with adenylyl imidodiphosphate (AMP-PNP), a non-hydrolysable analogue. CaMKII-dependent potentiation of channel opening resulted in significant increases in apparent steady-state open probability (P(o)) and sustained channel current at negative voltages. Under identical conditions, CaMKII activation did not regulate the activity of Ca(v)3.1 channels, the first cloned member (alpha1G) of the T-type Ca(2+) channel family. Our results provide the first evidence for the differential regulation of two members of the Ca(v)3 family by protein kinase activation and the first report reconstituting CaMKII-dependent regulation of any cloned Ca(2+) channel.
Figures

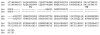

Similar articles
-
Stimulation of unitary T-type Ca(2+) channel currents by calmodulin-dependent protein kinase II.Am J Physiol Cell Physiol. 2000 Dec;279(6):C1694-703. doi: 10.1152/ajpcell.2000.279.6.C1694. Am J Physiol Cell Physiol. 2000. PMID: 11078683
-
Ca2+/calmodulin-dependent protein kinase II activation and regulation of adrenal glomerulosa Ca2+ signaling.Am J Physiol. 1995 Dec;269(6 Pt 2):F751-60. doi: 10.1152/ajprenal.1995.269.6.F751. Am J Physiol. 1995. PMID: 8594869
-
Molecular basis for the modulation of native T-type Ca2+ channels in vivo by Ca2+/calmodulin-dependent protein kinase II.J Clin Invest. 2006 Sep;116(9):2403-12. doi: 10.1172/JCI27918. Epub 2006 Aug 17. J Clin Invest. 2006. PMID: 16917542 Free PMC article.
-
Calmodulin and CaMKII as molecular switches for cardiac ion channels.Cardiovasc Res. 2007 Mar 1;73(4):641-7. doi: 10.1016/j.cardiores.2006.10.019. Epub 2006 Nov 10. Cardiovasc Res. 2007. PMID: 17137569 Review.
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
Cited by
-
Differential CaMKII regulation by voltage-gated calcium channels in the striatum.Mol Cell Neurosci. 2015 Sep;68:234-43. doi: 10.1016/j.mcn.2015.08.003. Epub 2015 Aug 5. Mol Cell Neurosci. 2015. PMID: 26255006 Free PMC article.
-
Ca(2+) calmodulin kinase and calcineurin mediate IGF-1-induced skeletal muscle dihydropyridine receptor alpha(1S) transcription.J Membr Biol. 2004 Jan 15;197(2):101-12. doi: 10.1007/s00232-003-0645-8. J Membr Biol. 2004. PMID: 15014912
-
Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-type calcium channel.J Neurosci. 2005 May 11;25(19):4844-55. doi: 10.1523/JNEUROSCI.0847-05.2005. J Neurosci. 2005. PMID: 15888660 Free PMC article.
-
A novel phospho-modulatory mechanism contributes to the calcium-dependent regulation of T-type Ca2+ channels.Sci Rep. 2019 Oct 30;9(1):15642. doi: 10.1038/s41598-019-52194-6. Sci Rep. 2019. PMID: 31666636 Free PMC article.
-
Effects of progesterone on T-type-Ca2+-channel expression in Purkinje cells.Neural Regen Res. 2022 Nov;17(11):2465-2471. doi: 10.4103/1673-5374.339008. Neural Regen Res. 2022. PMID: 35535898 Free PMC article.
References
-
- Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca2+-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circulation Research. 1994;75:854–861. - PubMed
-
- Barrett PQ, Isales CM, Bollag WB, McCarthy RT. Modulation of Ca2+ channels by atrial natriuretic peptide in the bovine adrenal glomerulosa cell. Canadian Journal of Physiology and Pharmacology. 1991;69:1553–1560. - PubMed
-
- Barrett PQ, Lu HK, Colbran R, Czernik A, Pancrazio JJ. Stimulation of unitary T-type Ca2+ channel currents by calmodulin-dependent protein kinase II. American Journal of Physiology. 2000;279:C1694–1703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

