Analysis of whole-cell currents by patch clamp of guinea-pig myenteric neurones in intact ganglia
- PMID: 11790812
- PMCID: PMC2290078
- DOI: 10.1113/jphysiol.2001.013051
Analysis of whole-cell currents by patch clamp of guinea-pig myenteric neurones in intact ganglia
Abstract
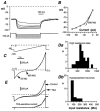
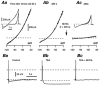
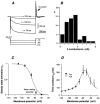
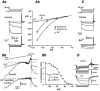
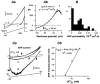
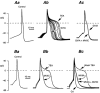
Whole-cell patch-clamp recordings taken from guinea-pig duodenal myenteric neurones within intact ganglia were used to determine the properties of S and AH neurones. Major currents that determine the states of AH neurones were identified and quantified. S neurones had resting potentials of -47 +/- 6 mV and input resistances (R(in)) of 713 +/- 49 MOmega at voltages ranging from -90 to -40 mV. At more negative levels, activation of a time-independent, caesium-sensitive, inward-rectifier current (I(Kir)) decreased R(in) to 103 +/- 10 MOmega. AH neurones had resting potentials of -57 +/- 4 mV and R(in) was 502 +/- 27 MOmega. R(in) fell to 194 +/- 16 MOmega upon hyperpolarization. This decrease was attributable mainly to the activation of a cationic h current, I(h), and to I(Kir). Resting potential and R(in) exhibited a low sensitivity to changes in [K(+)](o) in both AH and S neurones. This indicates that both cells have a low background K(+) permeability. The cationic current, I(h), contributed about 20 % to the resting conductance of AH neurones. It had a half-activation voltage of -72 +/- 2 mV, and a voltage sensitivity of 8.2 +/- 0.7 mV per e-fold change. I(h) has relatively fast, voltage-dependent kinetics, with on and off time constants in the range of 50-350 ms. AH neurones had a previously undescribed, low threshold, slowly inactivating, sodium-dependent current that was poorly sensitive to TTX. In AH neurones, the post-action-potential slow hyperpolarizing current, I(AHP), displayed large variation from cell to cell. I(AHP) appeared to be highly Ca(2+) sensitive, since its activation with either membrane depolarization or caffeine (1 mM) was not prevented by perfusing the cell with 10 mM BAPTA. We determined the identity of the Ca(2+) channels linked to I(AHP). Action potentials of AH neurones that were elongated by TEA (10 mM) were similarly shortened and I(AHP) was suppressed with each of the three omega-conotoxins GVIA, MVIIA and MVIIC (0.3-0.5 microM), but not with omega-agatoxin IVA (0.2 microM). There was no additivity between the effects of the three conotoxins, which indicates the presence of N- but not of P/Q-type Ca(2+) channels. A residual Ca(2+) current, resistant to all toxins, but blocked by 0.5 mM Cd(2+), could not generate I(AHP). This patch-clamp study, performed on intact ganglia, demonstrates that the AH neurones of the guinea-pig duodenum are under the control of four major currents, I(AHP), I(h), an N-type Ca(2+) current and a slowly inactivating Na(+) current.
Figures



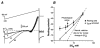

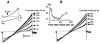
Similar articles
-
Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum.J Neurophysiol. 2001 May;85(5):1941-51. doi: 10.1152/jn.2001.85.5.1941. J Neurophysiol. 2001. PMID: 11353011
-
Action potential afterdepolarization mediated by a Ca2+-activated cation conductance in myenteric AH neurons.Neuroscience. 2002;115(2):375-93. doi: 10.1016/s0306-4522(02)00410-4. Neuroscience. 2002. PMID: 12421604
-
TEA- and apamin-resistant K(Ca) channels in guinea-pig myenteric neurons: slow AHP channels.J Physiol. 2002 Jan 15;538(Pt 2):421-33. doi: 10.1113/jphysiol.2001.012952. J Physiol. 2002. PMID: 11790810 Free PMC article.
-
Regulation of K+ channels underlying the slow afterhyperpolarization in enteric afterhyperpolarization-generating myenteric neurons: role of calcium and phosphorylation.Clin Exp Pharmacol Physiol. 2002 Oct;29(10):935-43. doi: 10.1046/j.1440-1681.2002.03755.x. Clin Exp Pharmacol Physiol. 2002. PMID: 12207575 Review.
-
Dorsal unpaired median neurones in the insect central nervous system: towards a better understanding of the ionic mechanisms underlying spontaneous electrical activity.J Exp Biol. 2000 Jun;203(Pt 11):1633-48. doi: 10.1242/jeb.203.11.1633. J Exp Biol. 2000. PMID: 10804154 Review.
Cited by
-
Identification of intrinsic primary afferent neurons in mouse jejunum.Neurogastroenterol Motil. 2020 Dec;32(12):e13989. doi: 10.1111/nmo.13989. Epub 2020 Sep 28. Neurogastroenterol Motil. 2020. PMID: 32986284 Free PMC article.
-
Squalamine reverses age-associated changes of firing patterns of myenteric sensory neurons and vagal fibres.Commun Biol. 2024 Jan 10;7(1):80. doi: 10.1038/s42003-023-05623-2. Commun Biol. 2024. PMID: 38200107 Free PMC article.
-
Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons.Nat Commun. 2013;4:1465. doi: 10.1038/ncomms2478. Nat Commun. 2013. PMID: 23403566
-
Isolation and transfection of myenteric neurons from mice for patch-clamp applications.Front Mol Neurosci. 2022 Dec 21;15:1076187. doi: 10.3389/fnmol.2022.1076187. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36618826 Free PMC article.
-
Morphine dependence in single enteric neurons from the mouse colon requires deletion of β-arrestin2.Physiol Rep. 2014 Sep 4;2(9):e12140. doi: 10.14814/phy2.12140. Print 2014 Sep 1. Physiol Rep. 2014. PMID: 25194025 Free PMC article.
References
-
- Baidan LV, Zholos AV, Shuba MF, Wood JD. Patch-clamp recording in myenteric neurones of guinea-pig small intestine. American Journal of Physiology. 1992a;262:G1074–1078. - PubMed
-
- Baidan LV, Zholos AV, Wood JD. Properties of calcium currents determined by patch-clamp recording in myenteric neurones from adult guinea-pig small intestine. Gastroenterology. 1992b;102:A420.
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

