Hypocretin-2 (orexin-B) modulation of superficial dorsal horn activity in rat
- PMID: 11790816
- PMCID: PMC2290067
- DOI: 10.1113/jphysiol.2001.013120
Hypocretin-2 (orexin-B) modulation of superficial dorsal horn activity in rat
Abstract
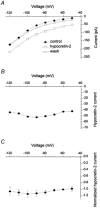
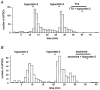
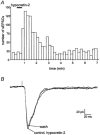
The hypothalamic peptides hypocretin-1 (orexin A) and hypocretin-2 (Hcrt-2; orexin B) are important in modulating behaviours demanding arousal, including sleep and appetite. Fibres containing hypocretin project from the hypothalamus to the superficial dorsal horn (SDH) of the spinal cord (laminae I and II); however, the effects produced by hypocretins on SDH neurones are unknown. To study the action of Hcrt-2 on individual SDH neurones, tight-seal, whole-cell recordings were made with biocytin-filled electrodes from rat lumbar spinal cord slices. In 19 of 63 neurones, Hcrt-2 (30 nM to 1 microM) evoked an inward (excitatory) current accompanied by an increase in baseline noise. The inward current and noise were unaffected by TTX but were blocked by the P(2X) purinergic receptor antagonist suramin (300-500 microM). Hcrt-2 (30 nM to 1 microM) increased the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in the majority of neurones. The sIPSC increase was blocked by strychnine (1 microM) and by TTX (1 microM), suggesting that the increased sIPSC frequency was glycine and action potential dependent. Hcrt-2 increased the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in a few neurones but had no effect on dorsal root-evoked EPSCs in these or in other neurones. Neurones located in outer lamina II, particularly radial and vertical cells, were most likely to respond to Hcrt-2. We conclude that Hcrt-2 has excitatory effects on certain SDH neurones, some of which exert inhibitory influences on other cells of the region, consistent with the perspective that hypocretin has a role in orchestrating reactions related to arousal, including nociception, pain and temperature sense.
Figures






References
-
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nature Neuroscience. 2001;4:72–77. - PubMed
-
- Bingham S, Davey PT, Babbs AJ, Irving EA, Sammons MJ, Wyles M, Jeffrey P, Cutler L, Riba I, Johns A, Porter RA, Upton N, Hunter AJ, Parsons AA. Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 2001;92:81–90. - PubMed
-
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammel T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. - PubMed
-
- de Lecea L, Kilduff T, Peyron C, Gao X-B, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FSII, Frankel WN, van ven Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the USA. 1998;95:322–327. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

