Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area
- PMID: 11790824
- PMCID: PMC2290077
- DOI: 10.1113/jphysiol.2001.012888
Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area
Abstract
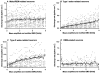
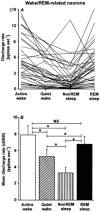
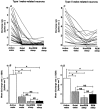
The perifornical lateral hypothalamic area (PF-LHA) has been implicated in the control of several waking behaviours, including feeding, motor activity and arousal. Several cell types are located in the PF-LHA, including projection neurons that contain the hypocretin peptides (also known as orexins). Recent findings suggest that hypocretin neurons are involved in sleep-wake regulation. Loss of hypocretin neurons in the human disorder narcolepsy is associated with excessive somnolence, cataplexy and increased propensity for rapid eye movement (REM) sleep. However, the relationship of PF-LHA neuronal activity to different arousal states is unknown. We recorded neuronal activity in the PF-LHA of rats during natural sleep and waking. Neuronal discharge rates were calculated during active waking (waking accompanied by movement), quiet waking, non-REM sleep and REM sleep. Fifty-six of 106 neurons (53 %) were classified as wake/REM-related. These neurons exhibited peak discharge rates during waking and REM sleep and reduced discharge rates during non-REM sleep. Wake-related neurons (38 %) exhibited reduced discharge rates during both non-REM and REM sleep when compared to that during waking. Wake-related neurons exhibited significantly higher discharge rates during active waking than during quiet waking. The discharge of wake-related neurons was positively correlated with muscle activity across all sleep-waking states. Recording sites were located within the hypocretin-immunoreactive neuronal field of the PF-LHA. Although the neurotransmitter phenotype of recorded cells was not determined, the prevalence of neurons with wake-related discharge patterns is consistent with the hypothesis that the PF-LHA participates in the regulation of arousal, muscle activity and sleep-waking states.
Figures





References
-
- Alam MN, McGinty D, Szymusiak R. Thermosensitive neurons of the diagonal band in rats: relation to wakefulness and non-rapid eye movement sleep. Brain Research. 1997;752:81–89. - PubMed
-
- Bittencourt JC, Frigo L, Rissman RA, Casatti CA, Nahon JL, Bauer JA. The distribution of melanin-concentrating hormone in the monkey brain (Cebus apella) Brain Research. 1998;804:140–143. - PubMed
-
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. Journal of Comparative Neurology. 1992;319:218–245. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

