Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells
- PMID: 11790825
- PMCID: PMC2290065
- DOI: 10.1113/jphysiol.2001.013222
Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells
Abstract
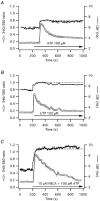
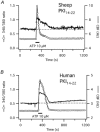
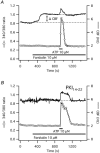
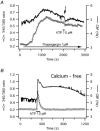
Stimulation of ovine airway epithelial cells with 10 microM ATP for 1 min at 25 degrees C transiently increased both cytoplasmic calcium (fura-2 epifluorescence microscopy) and ciliary beat frequency (CBF; differential interference contrast microscopy) with a similar time course. Identical purinergic stimulation of human airway epithelial cells at 25 or 35 degrees C, however, lead to an increase in CBF that outlasted the calcium transient at least 20 min. While a nitric oxide synthase inhibitor had no effect, pre-treatment of human cells with inhibitors of cAMP-dependent kinase (PKA), 10 microM myristoylated PKA-inhibitory peptide and 1 microM KT-5720, as well as an inhibitor of adenylyl cyclase, 1 mM SQ22536, blocked the prolonged, but not calcium-coupled CBF increase. Addition of PKA inhibitors after purinergic stimulation only partially reduced CBF from its elevated plateau. Prolonged CBF increases did not depend on adenosine production as 10 microM UTP had an effect similar to ATP and 8-sulphophenyl-theophylline did not block them. After increasing human CBF in a PKA-dependent manner to a stable plateau with forskolin (10 microM), ATP caused only a transient, calcium-coupled CBF increase. Calcium transients were necessary for both short-term and prolonged CBF changes as ATP failed to produce CBF increases after emptying calcium stores with 1 microM thapsigargin. These data suggest that in human, but not ovine airway epithelial cells, ATP-induced calcium transients activate a signalling cascade including adenylyl cyclase and PKA. The resulting prolonged CBF stimulation does not rely only on PKA activity, suggesting that the decay of CBF is influenced by ciliary phosphatase activity.
Figures







Similar articles
-
Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea.J Physiol. 1999 Apr 1;516 ( Pt 1)(Pt 1):179-90. doi: 10.1111/j.1469-7793.1999.179aa.x. J Physiol. 1999. PMID: 10066932 Free PMC article.
-
ATP regulation of ciliary beat frequency in rat tracheal and distal airway epithelium.Exp Physiol. 2005 Jul;90(4):535-44. doi: 10.1113/expphysiol.2004.028746. Epub 2005 Mar 15. Exp Physiol. 2005. PMID: 15769883
-
Regulation of human airway ciliary beat frequency by intracellular pH.J Physiol. 2004 Oct 15;560(Pt 2):519-32. doi: 10.1113/jphysiol.2004.068171. Epub 2004 Aug 12. J Physiol. 2004. PMID: 15308676 Free PMC article.
-
Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP.J Physiol. 2003 Feb 1;546(Pt 3):733-49. doi: 10.1113/jphysiol.2002.028704. J Physiol. 2003. PMID: 12563000 Free PMC article.
-
Respiratory Cilia as a Therapeutic Target of Phosphodiesterase Inhibitors.Front Pharmacol. 2020 May 6;11:609. doi: 10.3389/fphar.2020.00609. eCollection 2020. Front Pharmacol. 2020. PMID: 32435198 Free PMC article. Review.
Cited by
-
Identification of pannexins in rat nasal mucosa.Allergy Rhinol (Providence). 2013 Jan;4(2):63-65. doi: 10.2500/ar.2013.4.0052. Allergy Rhinol (Providence). 2013. PMID: 27792631 Free PMC article.
-
CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures.Mol Biol Cell. 2010 Aug 1;21(15):2639-48. doi: 10.1091/mbc.E09-12-1004. Epub 2010 Jun 16. Mol Biol Cell. 2010. PMID: 20554763 Free PMC article.
-
A fixed 20:1 combination of cafedrine/theodrenaline increases cytosolic Ca2+ concentration in human tracheal epithelial cells via ryanodine receptor-mediated Ca2+ release.Sci Rep. 2023 Sep 27;13(1):16216. doi: 10.1038/s41598-023-43342-0. Sci Rep. 2023. PMID: 37758747 Free PMC article.
-
Adenosine activation of A(2B) receptor(s) is essential for stimulated epithelial ciliary motility and clearance.Am J Physiol Lung Cell Mol Physiol. 2011 Aug;301(2):L171-80. doi: 10.1152/ajplung.00203.2010. Epub 2011 May 27. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21622845 Free PMC article.
-
A soluble adenylyl cyclase form targets to axonemes and rescues beat regulation in soluble adenylyl cyclase knockout mice.Am J Respir Cell Mol Biol. 2014 Dec;51(6):750-60. doi: 10.1165/rcmb.2013-0542OC. Am J Respir Cell Mol Biol. 2014. PMID: 24874272 Free PMC article.
References
-
- Adler KB, Cheng PW, Kim KC. Characterization of guinea pig tracheal epithelial cells maintained in biphasic organotypic culture: cellular composition and biochemical analysis of released glycoconjugates. American Journal of Respiratory Cell and Molecular Biology. 1990;2:145–154. - PubMed
-
- Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, William Davis C, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and muc5b are strongly induced. American Journal of Respiratory Cell and Molecular Biology. 1999;20:595–604. - PubMed
-
- Braiman A, Zagoory O, Priel Z. PKA induces Ca2+ release and enhances ciliary beat frequency in a Ca2+-dependent and -independent manner. American Journal of Physiology. 1998;275:C790–797. - PubMed
-
- Cabell L, Audesirk G. Effects of selective inhibition of protein kinase C, cyclic AMP-dependent protein kinase, and Ca2+-calmodulin-dependent protein kinase on neurite development in cultured rat hippocampal neurons. International Journal Developmental Neuroscience. 1993;11:357–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

