Crystal structure of the collagen triple helix model [(Pro-Pro-Gly)(10)](3)
- PMID: 11790836
- PMCID: PMC2373432
- DOI: 10.1110/ps.32602
Crystal structure of the collagen triple helix model [(Pro-Pro-Gly)(10)](3)
Abstract
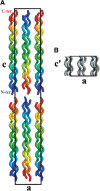
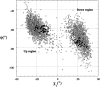
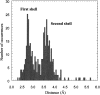
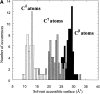
The first report of the full-length structure of the collagen-like polypeptide [(Pro-Pro-Gly)(10)](3) is given. This structure was obtained from crystals grown in a microgravity environment, which diffracted up to 1.3 A, using synchrotron radiation. The final model, which was refined to an R(factor) of 0.18, is the highest-resolution description of a collagen triple helix reported to date. This structure provides clues regarding a series of aspects related to collagen triple helix structure and assembly. The strict dependence of proline puckering on the position inside the Pro-Pro-Gly triplets and the correlation between backbone and side chain dihedral angles support the propensity-based mechanism of triple helix stabilization/destabilization induced by hydroxyproline. Furthermore, the analysis of [(Pro-Pro-Gly)(10)](3) packing, which is governed by electrostatic interactions, suggests that charges may act as locking features in the axial organization of triple helices in the collagen fibrils.
Figures






References
-
- Bella, J., Brodsky, B., and Berman, H.M. 1995. Hydration structure of a collagen peptide. Structure 3 893–906. - PubMed
-
- Bella, J., Eaton, M., Brodsky, B., and Berman, H.M. 1994. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science 266 75–81. - PubMed
-
- Berisio, R., Vitagliano, L., Mazzarella, L., and Zagari, A. 2001. Crystal structure determination of the collagen-like polypeptide with repeating sequence Pro-Hyp-Gly: Implications for hydration. Biopolymers 56 8–13. - PubMed
-
- Berisio, R., Vitagliano, L., Sorrentino, G., Carotenuto, L., Piccolo, C., Mazzarella, L., and Zagari, A. 2000. Effects of microgravity on the crystal quality of a collagen-like polypeptide. Acta Crystallogr. D 56 55–61. - PubMed
-
- Berman, H.M., Bhat, T.N., Bourne, P.E., Feng, Z., Gilliland, G., Weissig, H., and Westbrook, J. 2000. The Protein Data Bank and the challenge of structural genomics. Nat. Struct. Biol. 7 957–959. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

