Association of human aging with a functional variant of klotho
- PMID: 11792841
- PMCID: PMC117395
- DOI: 10.1073/pnas.022484299
Association of human aging with a functional variant of klotho
Abstract
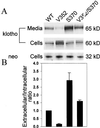
Mice deficient in Klotho gene expression exhibit a syndrome resembling premature human aging. To determine whether variation in the human KLOTHO locus contributes to survival, we applied two newly characterized polymorphic microsatellite markers flanking the gene in a population-based association study. In a cohort chosen for its homogeneity, Bohemian Czechs, we demonstrated significant differences in selected marker allele frequencies between newborn and elderly individuals (P < 0.05). These results precipitated a search for functional variants of klotho. We identified an allele, termed KL-VS, containing six sequence variants in complete linkage disequilibrium, two of which result in amino acid substitutions F352V and C370S. Homozygous elderly individuals were underrepresented in three distinct populations: Bohemian Czechs, Baltimore Caucasians, and Baltimore African-Americans [combined odds ratio (OR) = 2.59, P < 0.0023]. In a transient transfection assay, secreted levels of klotho harboring V352 are reduced 6-fold, whereas extracellular levels of the S370 form are increased 2.9-fold. The V352/S370 double mutant exhibits an intermediate phenotype (1.6-fold increase), providing a rare example of intragenic complementation in cis by human single nucleotide polymorphisms. The remarkable conservation of F352 among homologous proteins suggests that it is functionally important. The corresponding substitution, F289V, in the closest human klotho paralog with a known substrate, cBGL1, completely eliminates its ability to cleave p-nitrophenyl-beta-D-glucoside. These results suggest that the KL-VS allele influences the trafficking and catalytic activity of klotho, and that variation in klotho function contributes to heterogeneity in the onset and severity of human age-related phenotypes.
Figures


References
-
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Nature (London) 1997;390:45–51. - PubMed
-
- Mian I S. Blood Cells Mol Dis. 1998;24:83–100. - PubMed
-
- Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Biochem Biophys Res Commun. 1998;242:626–630. - PubMed
-
- Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. Biochem Biophys Res Commun. 2000;276:767–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

