Heat shock protein 90 catalyzes activation of the prekallikrein-kininogen complex in the absence of factor XII
- PMID: 11792853
- PMCID: PMC117402
- DOI: 10.1073/pnas.022626899
Heat shock protein 90 catalyzes activation of the prekallikrein-kininogen complex in the absence of factor XII
Abstract
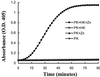
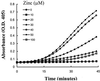
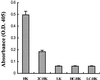
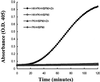
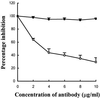
Bradykinin is a major mediator of swelling in C1 inhibitor deficiency as well as the angioedema seen with ACE inhibitors and may contribute to bronchial hyperreactivity in asthma. Formation of bradykinin occurs in the fluid phase and along cell surfaces requiring interaction of factor XII, prekallikrein, and high M(r) kininogen (HK). Recent data suggest that activation of the kinin-forming cascade can occur on the surface of endothelial cells, even in the absence of factor XII. We sought to further define this factor XII-independent mechanism of kinin formation. Both cytosolic and membrane fractions from endothelial cells possessed the ability to catalyze prekallikrein conversion to kallikrein, and activation depended on the presence of HK and zinc ion. We fractionated the cytosol by ion exchange chromatography and affinity chromatography by using corn trypsin inhibitor as ligand. The fractions with peak activity were subjected to SDS gel electrophoresis and ligand blot with biotinylated corn trypsin inhibitor, and positive bands were sequenced. Heat shock protein 90 (Hsp90) was identified as the protein responsible for zinc-dependent prekallikrein activation in the presence of HK. Zinc-dependent activation of the prekallikrein-HK complex also depended on addition of either alpha and beta isoforms of Hsp90 and the activation on endothelial cells was inhibited on addition of polyclonal Ab to Hsp90 in a dose-dependent manner. Although the mechanism by which Hsp90 activates the kinin-forming cascade is not understood, this protein represents the cellular contribution to the reaction and may become the dominant mechanism in pathologic circumstances in which Hsp90 is highly expressed or secreted.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

