Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases
- PMID: 11796357
- PMCID: PMC127070
- DOI: 10.1128/AAC.46.2.451-457.2002
Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases
Abstract
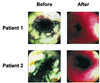
Caspofungin is an antifungal agent of the novel echinocandin class. We investigated its efficacy, safety, and tolerability as therapy for oropharyngeal and/or esophageal candidiasis in a phase II dose-ranging study. Patients were randomized in a double-blind manner to receive either caspofungin acetate (35, 50, or 70 mg) or amphotericin B (0.5 mg/kg of body weight) intravenously once daily for 7 to 14 days. A favorable response required both complete resolution of symptoms and quantifiable improvement of mucosal lesions 3 to 4 days after discontinuation of study drug. Efficacy was assessed using a modified intent-to-treat analysis. No hypothesis testing of efficacy was planned or performed. Of 140 enrolled patients, 63% had esophageal involvement and 98% were infected with the human immunodeficiency virus (HIV) (median CD4 count, 30/mm(3)). A modestly higher proportion of patients in each of the caspofungin groups (74 to 91%) achieved favorable responses compared to amphotericin B recipients (63%), but there was considerable overlap in the 95% confidence intervals surrounding these point estimates. Similar trends were found in the subgroups with esophageal involvement, a history of fluconazole failure, and CD4 counts of < or =50/mm(3). A smaller proportion of patients receiving any dose of caspofungin experienced drug-related adverse events compared to patients given standard doses of conventional amphotericin B (P < 0.01). Caspofungin provided a generally well-tolerated parenteral therapeutic option for HIV-infected patients with oropharyngeal and/or esophageal candidiasis in this study.
Figures
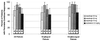

References
-
- Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamine-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. - PMC - PubMed
-
- Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, et al. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. - PMC - PubMed
-
- Andriole, V. T. 1999. Current and future antifungal therapy: new targets for antifungal agents. J. Antimicrob. Chemother. 44:151-162. - PubMed
-
- Baehr, P. H., and G. B. McDonald. 1994. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 106:509-532. - PubMed
-
- Barchiesi, F., A. L. Colombo, D. A. McGough, A. W. Fothergill, and M. G. Rinaldi. 1994. In vitro activity of itraconazole against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 38:1530-1533. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

