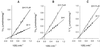Stability of new carbapenem DA-1131 to renal dipeptidase (dehydropeptidase I)
- PMID: 11796382
- PMCID: PMC127065
- DOI: 10.1128/AAC.46.2.575-577.2002
Stability of new carbapenem DA-1131 to renal dipeptidase (dehydropeptidase I)
Abstract
The stability of DA-1131 to renal dipeptidase (RDPase) (EC 3.4.13.19) was compared with that of imipenem and meropenem by V(max)/K(m) ratios as an index of the enzyme's preference for substrates. Our results showed a decreasing order of imipenem (6.24), meropenem (2.41), and DA-1131 (1.39). The biochemical evaluation of DA-1131 as the least preferred substrate of RDPase suggests its potential use as a novel beta-lactam antibiotic which may be usable without coadministration of RDPase inhibitors once its clinical suitability is proven.
Figures
References
-
- Blumer, J. L. 1997. Meropenem: evaluation of a new generation carbapenem. Int. J. Microb. Agents 8:73-92. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Campbell, B. J., Y. D. Shih, L. J. Forrester, and W. L. Zahler. 1988. Specificity and inhibition studies of human renal dipeptidase. Biochim. Biophys. Acta 956:110-118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources