Fibroblasts from the inner granulation tissue of the pseudocapsule in hips at revision arthroplasty induce osteoclast differentiation, as do stromal cells
- PMID: 11796394
- PMCID: PMC1753995
- DOI: 10.1136/ard.61.2.103
Fibroblasts from the inner granulation tissue of the pseudocapsule in hips at revision arthroplasty induce osteoclast differentiation, as do stromal cells
Abstract
Background: It has previously been shown that many osteoclast precursors are included in the granulation tissue within the pseudocapsule obtained at revision arthroplasty from hips with osteolysis. In vitro culture of only cells isolated from the granulation tissue has been previously shown to generate many mature osteoclasts.
Objective: To investigate the presence or otherwise of supporting cells, similar to stromal cells, which differentiate osteoclasts within the granulation tissue.
Methods: Cells isolated from the granulation tissue were cultured alone, and after four weeks fibroblast-like cells (granulation fibroblasts) remained. Rat non-adherent bone marrow cells (NA-BMCs) were co-cultured with the granulation fibroblasts with or without 1alpha,25(OH)2D3 (10(-8) M) or heat treated ROS 17/2.8 cell conditioned medium (ht ROSCM), or both. Multinucleated cells (MNCs), which formed, were assessed by biochemical and functional characterisation of osteoclasts. Receptor activator of NFkappaB ligand (RANKL) was investigated by immunohistochemistry.
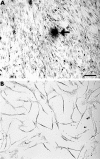
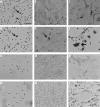
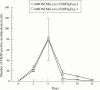
Results: Co-culture of NA-BMCs and granulation fibroblasts caused the formation of tartrate resistant acid phosphatase (TRAP) positive MNCs, which had the calcitonin receptor (CTR), the Kat-1 antigen, which is specific to the surface of rat osteoclasts, and the ability to form pits in the presence of both 1alpha,25(OH)2D3 and ht ROSCM or in the presence of just ht ROSCM. RANKL was detected in fibroblast-like cells in the granulation tissue.
Conclusion: These data suggest that granulation fibroblasts support osteoclast differentiation, as do osteoblasts/stromal cells, and may play a part in aseptic loosening.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

