Differences in components at delayed-type hypersensitivity reaction sites in mice immunized with either a protective or a nonprotective immunogen of Cryptococcus neoformans
- PMID: 11796587
- PMCID: PMC127722
- DOI: 10.1128/IAI.70.2.591-600.2002
Differences in components at delayed-type hypersensitivity reaction sites in mice immunized with either a protective or a nonprotective immunogen of Cryptococcus neoformans
Abstract
Cell-mediated immunity is the major protective mechanism against Cryptococcus neoformans. Delayed swelling reactions, i.e., delayed-type hypersensitivity (DTH), in response to an intradermal injection of specific antigen are used as a means of detecting a cell-mediated immune (CMI) response to the antigen. We have found previously that the presence of an anticryptococcal DTH response in mice is not always indicative of protection against a cryptococcal infection. Using one immunogen that induces a protective anticryptococcal CMI response and one that induces a nonprotective response, we have shown that mice immunized with the protective immunogen undergo a classical DTH response characterized by mononuclear cell and neutrophil infiltrates and the presence of gamma interferon and NO. In contrast, immunization with the nonprotective immunogen results in an influx of primarily neutrophils and production of tumor necrosis factor alpha (TNF-alpha) at the DTH reaction site. Even when the anticryptococcal DTH response was augmented by blocking the down-regulator, CTLA-4 (CD152), on T cells in the mice given the nonprotective immunogen, the main leukocyte population infiltrating the DTH reaction site is the neutrophil. Although TNF-alpha is increased at the DTH reaction site in mice immunized with the nonprotective immunogen, it is unlikely that TNF-alpha activates the neutrophils, because the density of TNF receptors on the neutrophils is reduced below control levels. Uncoupling of DTH reactivity and protection has been demonstrated in other infectious-disease models; however, the mechanisms differ from our model. These findings stress the importance of defining the cascade of events occurring in response to various immunogens and establishing the relationships between protection and DTH reactions.
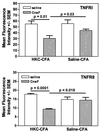
Figures





Similar articles
-
Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses.J Immunol. 2000 Jul 1;165(1):158-67. doi: 10.4049/jimmunol.165.1.158. J Immunol. 2000. PMID: 10861048
-
Effects of tumor necrosis factor alpha on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response.Infect Immun. 2003 Jan;71(1):68-74. doi: 10.1128/IAI.71.1.68-74.2003. Infect Immun. 2003. PMID: 12496150 Free PMC article.
-
CTLA-4 down-regulates the protective anticryptococcal cell-mediated immune response.Infect Immun. 2000 Aug;68(8):4624-30. doi: 10.1128/IAI.68.8.4624-4630.2000. Infect Immun. 2000. PMID: 10899865 Free PMC article.
-
MIP-1 alpha contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans.J Leukoc Biol. 1997 Feb;61(2):147-55. doi: 10.1002/jlb.61.2.147. J Leukoc Biol. 1997. PMID: 9021919
-
Polysaccharide antigens of the capsule of Cryptococcus neoformans.Infect Immun. 1994 May;62(5):1507-12. doi: 10.1128/iai.62.5.1507-1512.1994. Infect Immun. 1994. PMID: 8168912 Free PMC article. Review.
Cited by
-
Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions.Infect Immun. 2010 Apr;78(4):1601-9. doi: 10.1128/IAI.01171-09. Epub 2010 Feb 9. Infect Immun. 2010. PMID: 20145096 Free PMC article.
-
Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-Cell responses.Infect Immun. 2002 Oct;70(10):5485-93. doi: 10.1128/IAI.70.10.5485-5493.2002. Infect Immun. 2002. PMID: 12228274 Free PMC article.
-
Protective efficacy of antigenic fractions in mouse models of cryptococcosis.Infect Immun. 2004 Mar;72(3):1746-54. doi: 10.1128/IAI.72.3.1746-1754.2004. Infect Immun. 2004. PMID: 14977983 Free PMC article.
-
Make It Simple: (SR-A1+TLR7) Macrophage Targeted NANOarchaeosomes.Front Bioeng Biotechnol. 2018 Nov 6;6:163. doi: 10.3389/fbioe.2018.00163. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30460231 Free PMC article.
-
Type I Natural Killer T Cells as Key Regulators of the Immune Response to Infectious Diseases.Clin Microbiol Rev. 2020 Dec 23;34(2):e00232-20. doi: 10.1128/CMR.00232-20. Print 2021 Mar 17. Clin Microbiol Rev. 2020. PMID: 33361143 Free PMC article. Review.
References
-
- Annie, T., T. Fong, and T. R. Mosmann. 1989. The role of IFNγ in delayed-type hypersensitivity mediated by Th1 clones. J. Immunol. 143:2887-2899. - PubMed
-
- Bauman, S. K., K. L. Nichols, and J. W. Murphy. 2000. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 165:158-167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

