Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae
- PMID: 11796608
- PMCID: PMC127731
- DOI: 10.1128/IAI.70.2.749-761.2002
Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae
Abstract
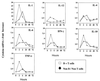
Proinflammatory cytokines play a critical role in innate host defense against extracellular bacteria. However, little is known regarding the effects of these cytokines on the adaptive humoral response. Mice injected with a neutralizing anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody (MAb) at the time of primary immunization with intact Streptococcus pneumoniae (strain R36A) showed a substantial reduction in both the primary immunoglobulin G (IgG) response specific for the cell wall protein, pneumococcal surface protein A (PspA), as well as in the development of PspA-specific memory. In contrast, anti-TNF-alpha MAb injected only at the time of secondary immunization with R36A failed to alter the boosted anti-PspA response. TNF-alpha was required only within the first 48 to 72 h after primary immunization with R36A and was induced both by non-B and non-T cells and by lymphoid cells, within 2 to 6 h after immunization, with levels returning to normal by 24 h. Thus, the early innate release of TNF-alpha was critical for optimal stimulation of the subsequent adaptive humoral response to R36A. Additional proinflammatory (interleukin 1 [IL-1], IL-6, IL-12, and gamma interferon [IFN-gamma]) as well as anti-inflammatory (IL-4 and IL-10) cytokines were also transiently induced. Mice genetically deficient in IL-6, IFN-gamma, or IL-12 also showed a reduced IgG anti-PspA response of all IgG isotypes. In contrast, IL-4(-/-) and IL-10(-/-) mice immunized with R36A showed a significant elevation in the IgG anti-PspA response, except that there was decreased IgG1 in IL-4(-/-) mice. In this regard, a marked enhancement in the induction of proinflammatory cytokines was observed in the absence of IL-10, relative to controls. Ig isotype titers specific for the phosphorycholine determinant of C-polysaccharide were similarly regulated, but to a much more modest degree. These data suggest that proinflammatory and anti-inflammatory cytokines differentially regulate an in vivo protein- and polysaccharide-specific Ig response to an extracellular bacteria.
Figures








References
-
- Abrams, J. S., M. G. Roncarolo, H. Yssel. U. Andersson, G. J. Gleich, and J. E. Silver. 1992. Strategies for anticytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol. Rev. 127:5-24. - PubMed
-
- Akira, S. 2000. Toll-like receptors: lessons from knockout mice. Biochem. Soc. Trans. 28:551-556. - PubMed
-
- Arase, H., N. Arase, K.-I. Nakagawa, R. A. Good, and K. Onoe. 1993. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur. J. Immunol. 23:307-310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

