Fas ligand-expressing B-1a lymphocytes mediate CD4(+)-T-cell apoptosis during schistosomal infection: induction by interleukin 4 (IL-4) and IL-10
- PMID: 11796615
- PMCID: PMC127725
- DOI: 10.1128/IAI.70.2.812-819.2002
Fas ligand-expressing B-1a lymphocytes mediate CD4(+)-T-cell apoptosis during schistosomal infection: induction by interleukin 4 (IL-4) and IL-10
Abstract
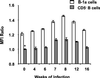
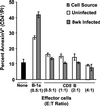
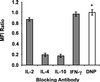
A previous study of the murine model of Schistosoma mansoni infection has implicated splenic CD19(+) B lymphocytes as Fas ligand (FasL)-bearing mediators of CD4(+) T-lymphocyte apoptosis. The present study shows that B-cell deficiency leads to decreased CD4(+) T-cell apoptosis during infection and compares FasL expression and killer function of B-1a- and CD5(-) B-lymphocyte subsets. B-1a cells from uninfected mice displayed constitutive expression of FasL compared with that of CD5(-) B cells. FasL expression was enhanced following worm egg deposition and antigenic stimulation on both subsets of B cells. Purified B-1a cells from uninfected mice were potent effectors of CD4(+) T-cell apoptosis, and the killing effect was enhanced during schistosome infection. FasL expression by splenic B cells required CD4(+)-T-cell help that was replaced by addition of culture supernatants from antigen-stimulated splenocytes of infected mice. The culture-supernatant-stimulated FasL expression was inhibited by anti-interleukin 10 (IL-10) and anti-IL-4 antibodies. Culture of purified B cells with recombinant IL-4 (rIL-4), rIL-10, and soluble egg antigens (SEA) led to increased expression of FasL on B-1a cells. These results suggest that FasL-expressing, splenic B-1a cells are important mediators of SEA-stimulated CD4(+)-T-cell apoptosis and that maximal FasL expression on B-1a cells is dependent on antigenic stimulation and the presence of IL-4 and IL-10.
Figures
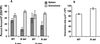

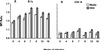
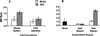
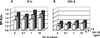
Similar articles
-
Soluble egg antigen-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL(+) T and B cells during murine Schistosoma mansoni infection.Infect Immun. 2001 Jan;69(1):271-80. doi: 10.1128/IAI.69.1.271-280.2001. Infect Immun. 2001. PMID: 11119515 Free PMC article.
-
Reduced Fas ligand-expressing splenic CD5+ B lymphocytes in severe collagen-induced arthritis.Arthritis Res Ther. 2009;11(4):R128. doi: 10.1186/ar2795. Epub 2009 Aug 25. Arthritis Res Ther. 2009. PMID: 19706160 Free PMC article.
-
Apoptosis by neglect of CD4+ Th cells in granulomas: a novel effector mechanism involved in the control of egg-induced immunopathology in murine schistosomiasis.J Immunol. 2003 Aug 15;171(4):1859-67. doi: 10.4049/jimmunol.171.4.1859. J Immunol. 2003. PMID: 12902487
-
Regulatory B cells as inhibitors of immune responses and inflammation.Immunol Rev. 2008 Aug;224:201-14. doi: 10.1111/j.1600-065X.2008.00661.x. Immunol Rev. 2008. PMID: 18759928 Review.
-
Killer B lymphocytes: the evidence and the potential.Inflamm Res. 2009 Jul;58(7):345-57. doi: 10.1007/s00011-009-0014-x. Epub 2009 Mar 5. Inflamm Res. 2009. PMID: 19262989 Free PMC article. Review.
Cited by
-
Killer B lymphocytes and their fas ligand positive exosomes as inducers of immune tolerance.Front Immunol. 2015 Mar 20;6:122. doi: 10.3389/fimmu.2015.00122. eCollection 2015. Front Immunol. 2015. PMID: 25852690 Free PMC article.
-
Expression of Tumor Necrosis Factor Receptor 2 Characterizes TLR9-Driven Formation of Interleukin-10-Producing B Cells.Front Immunol. 2018 Jan 19;8:1951. doi: 10.3389/fimmu.2017.01951. eCollection 2017. Front Immunol. 2018. PMID: 29403470 Free PMC article.
-
Regulatory B cells: TIM-1, transplant tolerance, and rejection.Immunol Rev. 2021 Jan;299(1):31-44. doi: 10.1111/imr.12933. Epub 2021 Jan 22. Immunol Rev. 2021. PMID: 33484008 Free PMC article. Review.
-
The expanding family of regulatory B cells.Int Immunol. 2015 Oct;27(10):479-86. doi: 10.1093/intimm/dxv038. Epub 2015 Jun 12. Int Immunol. 2015. PMID: 26071023 Free PMC article. Review.
-
The Dynamics of B Cell Aging in Health and Disease.Front Immunol. 2021 Oct 5;12:733566. doi: 10.3389/fimmu.2021.733566. eCollection 2021. Front Immunol. 2021. PMID: 34675924 Free PMC article. Review.
References
-
- Boros, D. L. 1999. T helper cell populations, cytokine dynamics, and pathology of the schistosome egg granuloma. Microbes Infect. 1:511-516. - PubMed
-
- Boros, D. L., A. F. Amsden, and A. T. Hood. 1982. Modulation of granulomatous hypersensitivity. IV. Immunoglobulin and antibody production by vigorous and immunomodulated liver granulomas of Schistosoma mansoni-infected mice. J. Immunol. 128:1050-1053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

