Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces
- PMID: 11796623
- PMCID: PMC127692
- DOI: 10.1128/IAI.70.2.878-888.2002
Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces
Abstract
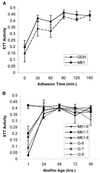
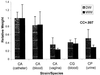
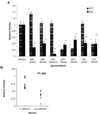
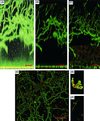
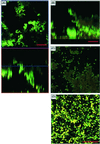
Little is known about fungal biofilms, which may cause infection and antibiotic resistance. In this study, biofilm formation by different Candida species, particularly Candida albicans and C. parapsilosis, was evaluated by using a clinically relevant model of Candida biofilm on medical devices. Candida biofilms were allowed to form on silicone elastomer and were quantified by tetrazolium (XTT) and dry weight (DW) assays. Formed biofilm was visualized by using fluorescence microscopy and confocal scanning laser microscopy with Calcofluor White (Sigma Chemical Co., St. Louis, Mo.), concanavalin A-Alexafluor 488 (Molecular Probes, Eugene, Oreg.), and FUN-1 (Molecular Probes) dyes. Although minimal variations in biofilm production among invasive C. albicans isolates were seen, significant differences between invasive and noninvasive isolates (P < 0.001) were noted. C. albicans isolates produced more biofilm than C. parapsilosis, C. glabrata, and C. tropicalis isolates, as determined by DW assays (P was <0.001 for all comparisons) and microscopy. Interestingly, noninvasive isolates demonstrated a higher level of XTT activity than invasive isolates. On microscopy, C. albicans biofilms had a morphology different from that of other species, consisting of a basal blastospore layer with a dense overlying matrix composed of exopolysaccharides and hyphae. In contrast, C. parapsilosis biofilms had less volume than C. albicans biofilms and were comprised exclusively of clumped blastospores. Unlike planktonically grown cells, Candida biofilms rapidly (within 6 h) developed fluconazole resistance (MIC, >128 microg/ml). Importantly, XTT and FUN-1 activity showed biofilm cells to be metabolically active. In conclusion, our data show that C. albicans produces quantitatively larger and qualitatively more complex biofilms than other species, in particular, C. parapsilosis.
Figures
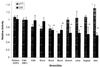

References
-
- Anaissie, E. J., J. H. Rex, O. Uzon, and S. Vartivarian. 1998. Predictors of adverse outcome in cancer patients with candidemia. Am. J. Med. 104:238-245. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48:671-679. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. - PubMed
-
- Banejee, S. N., G. T. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, et al. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. Am. J. Med. 91(Suppl. 3B):86-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

