Transcriptional regulators Cph1p and Efg1p mediate activation of the Candida albicans virulence gene SAP5 during infection
- PMID: 11796627
- PMCID: PMC127704
- DOI: 10.1128/IAI.70.2.921-927.2002
Transcriptional regulators Cph1p and Efg1p mediate activation of the Candida albicans virulence gene SAP5 during infection
Abstract
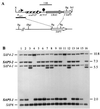
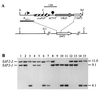
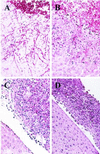
The opportunistic fungal pathogen Candida albicans can cause superficial as well as systemic infections. Successful adaptation to the different host niches encountered during infection requires coordinated expression of various virulence traits, including the switch between yeast and hyphal growth forms and secretion of aspartic proteinases. Using an in vivo expression technology that is based on genetic recombination as a reporter of gene activation during experimental candidiasis in mice, we investigated whether two signal transduction pathways controlling hyphal growth, a mitogen-activated protein kinase cascade ending in the transcriptional activator Cph1p and a cyclic AMP-dependent regulatory pathway that involves the transcription factor Efg1p, also control expression of the SAP5 gene, which encodes one of the secreted aspartic proteinases and is induced by host signals soon after infection. Our results show that both transcriptional regulators are important for SAP5 activation in vivo. SAP5 expression was reduced in a cph1 mutant, although filamentous growth in infected tissue was not detectably impaired. SAP5 expression was also reduced, but not eliminated, in an efg1 null mutant, although this strain grew exclusively in the yeast form in infected tissue, demonstrating that in contrast to in vitro conditions, SAP5 activation during infection does not depend on growth of C. albicans in the hyphal form. In a cph1 efg1 double mutant, however, SAP5 expression in infected mice was almost completely eliminated, suggesting that the two signal transduction pathways are important for SAP5 expression in vivo. The avirulence of the cph1 efg1 mutant seemed to be caused not only by the inability to form hyphae but also by a loss of expression of additional virulence genes in the host.
Figures

Similar articles
-
Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs.Infect Immun. 2002 Jul;70(7):3689-700. doi: 10.1128/IAI.70.7.3689-3700.2002. Infect Immun. 2002. PMID: 12065511 Free PMC article.
-
Reduced expression of the hyphal-independent Candida albicans proteinase genes SAP1 and SAP3 in the efg1 mutant is associated with attenuated virulence during infection of oral epithelium.J Med Microbiol. 2003 Aug;52(Pt 8):623-632. doi: 10.1099/jmm.0.05125-0. J Med Microbiol. 2003. PMID: 12867554
-
Profile of Candida albicans-secreted aspartic proteinase elicited during vaginal infection.Infect Immun. 2005 Mar;73(3):1828-35. doi: 10.1128/IAI.73.3.1828-1835.2005. Infect Immun. 2005. PMID: 15731084 Free PMC article.
-
Transcriptional control of hyphal morphogenesis in Candida albicans.FEMS Yeast Res. 2020 Feb 1;20(1):foaa005. doi: 10.1093/femsyr/foaa005. FEMS Yeast Res. 2020. PMID: 31981355 Free PMC article. Review.
-
Signaling through protein kinases and transcriptional regulators in Candida albicans.Crit Rev Microbiol. 2003;29(3):259-75. doi: 10.1080/713610451. Crit Rev Microbiol. 2003. PMID: 14582620 Review.
Cited by
-
Effect of the echinocandin caspofungin on expression of Candida albicans secretory aspartyl proteinases and phospholipase in vitro.Antimicrob Agents Chemother. 2002 Sep;46(9):3096-100. doi: 10.1128/AAC.46.9.3096-3100.2002. Antimicrob Agents Chemother. 2002. PMID: 12183282 Free PMC article.
-
Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions.Cell Microbiol. 2008 Nov;10(11):2180-96. doi: 10.1111/j.1462-5822.2008.01198.x. Epub 2008 Jul 4. Cell Microbiol. 2008. PMID: 18627379 Free PMC article.
-
Gliotoxin in Aspergillus fumigatus: an example that mycotoxins are potential virulence factors.Mycotoxin Res. 2009 Oct;25(3):123-31. doi: 10.1007/s12550-009-0020-4. Epub 2009 Aug 11. Mycotoxin Res. 2009. PMID: 23605091
-
Self-regulation of Candida albicans population size during GI colonization.PLoS Pathog. 2007 Dec;3(12):e184. doi: 10.1371/journal.ppat.0030184. PLoS Pathog. 2007. PMID: 18069889 Free PMC article.
-
Efg1 Controls caspofungin-induced cell aggregation of Candida albicans through the adhesin Als1.Eukaryot Cell. 2011 Dec;10(12):1694-704. doi: 10.1128/EC.05187-11. Epub 2011 Oct 28. Eukaryot Cell. 2011. PMID: 22037180 Free PMC article.
References
-
- Borg-von Zepelin, M., S. Beggah, K. Boggian, D. Sanglard, and M. Monod. 1998. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol. Microbiol. 28:543–554. - PubMed
-
- Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327–335. - PubMed
-
- Cutler, J. E. 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45:187–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

