In vivo modifications of small GTPase Rac and Cdc42 by Bordetella dermonecrotic toxin
- PMID: 11796639
- PMCID: PMC127728
- DOI: 10.1128/IAI.70.2.998-1001.2002
In vivo modifications of small GTPase Rac and Cdc42 by Bordetella dermonecrotic toxin
Abstract
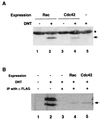
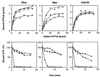
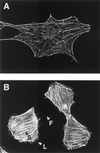
Bordetella dermonecrotic toxin (DNT) is known to activate the small GTPase Rho through deamidation or polyamination. In this study, we examined whether Rac and Cdc42, the two other members of the Rho family, serve as intracellular targets for the toxin. Immunoprecipitation and immunoblot assays revealed that DNT deamidated or polyaminated intracellular Rac and Cdc42. After the modifications, both Rac and Cdc42 lost their GTP-hydrolyzing, but not GTP-binding, activities. The interactions of the modified Rac and Cdc42 with their respective effectors were strictly dependent on GTP. MC3T3-E1 cells treated with DNT at high concentrations demonstrated extensive formations of lamellipodia and filopodia, which indicate the intracellular activation of Rac and Cdc42, respectively.
Figures

Similar articles
-
Identification of the C-terminal part of Bordetella dermonecrotic toxin as a transglutaminase for rho GTPases.J Biol Chem. 1999 Nov 5;274(45):31875-81. doi: 10.1074/jbc.274.45.31875. J Biol Chem. 1999. PMID: 10542213
-
Activation of rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin.EMBO J. 2000 Feb 15;19(4):521-30. doi: 10.1093/emboj/19.4.521. EMBO J. 2000. PMID: 10675321 Free PMC article.
-
Lysine and polyamines are substrates for transglutamination of Rho by the Bordetella dermonecrotic toxin.Infect Immun. 2001 Dec;69(12):7663-70. doi: 10.1128/IAI.69.12.7663-7670.2001. Infect Immun. 2001. PMID: 11705946 Free PMC article.
-
Escherichia coli cytotoxic necrotizing factors and Bordetella dermonecrotic toxin: the dermonecrosis-inducing toxins activating Rho small GTPases.Toxicon. 2001 Nov;39(11):1619-27. doi: 10.1016/s0041-0101(01)00149-0. Toxicon. 2001. PMID: 11595625 Review.
-
Bordetella dermonecrotic toxin exerting toxicity through activation of the small GTPase Rho.J Biochem. 2004 Oct;136(4):415-9. doi: 10.1093/jb/mvh155. J Biochem. 2004. PMID: 15625308 Review.
Cited by
-
Bordetella Dermonecrotic Toxin Is a Neurotropic Virulence Factor That Uses CaV3.1 as the Cell Surface Receptor.mBio. 2020 Mar 24;11(2):e03146-19. doi: 10.1128/mBio.03146-19. mBio. 2020. PMID: 32209694 Free PMC article.
-
Cellular and molecular action of the mitogenic protein-deamidating toxin from Pasteurella multocida.FEBS J. 2011 Dec;278(23):4616-32. doi: 10.1111/j.1742-4658.2011.08158.x. Epub 2011 May 31. FEBS J. 2011. PMID: 21569202 Free PMC article. Review.
-
Transglutaminase in Receptor and Neurotransmitter-Regulated Functions.Med One. 2018;3(6):e180012. doi: 10.20900/mo.20180012. Epub 2018 Dec 5. Med One. 2018. PMID: 30701197 Free PMC article.
-
Mycobacterium avium genes MAV_5138 and MAV_3679 are transcriptional regulators that play a role in invasion of epithelial cells, in part by their regulation of CipA, a putative surface protein interacting with host cell signaling pathways.J Bacteriol. 2009 Feb;191(4):1132-42. doi: 10.1128/JB.01359-07. Epub 2008 Dec 5. J Bacteriol. 2009. PMID: 19060135 Free PMC article.
-
Hierarchical determinants in cytotoxic necrotizing factor (CNF) toxins driving Rho G-protein deamidation versus transglutamination.mBio. 2024 Jul 17;15(7):e0122124. doi: 10.1128/mbio.01221-24. Epub 2024 Jun 26. mBio. 2024. PMID: 38920360 Free PMC article.
References
-
- Honda, A., M. Nogami, T. Yokozeki, M. Yamazaki, H. Nakamura, H. Watanabe, K. Kawamoto, K. Nakayama, A. J. Morris, M. A. Frohman, and Y. Kanaho. 1999. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein Arf6 in membrane ruffle formation. Cell 99:521-532. - PubMed
-
- Horiguchi, Y., N. Inoue, M. Masuda, T. Kashimoto, J. Katahira, N. Sugimoto, and M. Matsuda. 1997. Bordetella bronchiseptica dermonecrotizing toxin induces reorganization of actin stress fibers through deamidation of Gln-63 of the GTP-binding protein Rho. Proc. Natl. Acad. Sci. USA 94:11623-11626. - PMC - PubMed
-
- Horiguchi, Y., T. Nakai, and K. Kume. 1990. Simplified procedure for purification of Bordetella bronchiseptica dermonecrotic toxin. FEMS Microbiol. Lett. 66:39-43. - PubMed
-
- Horiguchi, Y., T. Okada, N. Sugimoto, Y. Morikawa, J. Katahira, and M. Matsuda. 1995. Effects of Bordetella bronchiseptica dermonecrotizing toxin on bone formation in calvaria of neonatal rats. FEMS Immunol. Med. Microbiol. 12:29-32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

