Effect of latent human immunodeficiency virus infection on cell surface phenotype
- PMID: 11799162
- PMCID: PMC135922
- DOI: 10.1128/jvi.76.4.1673-1681.2002
Effect of latent human immunodeficiency virus infection on cell surface phenotype
Abstract
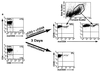
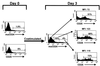
Highly active antiretroviral therapy has succeeded in many cases in suppressing virus production in patients infected with human immunodeficiency virus (HIV); however, once treatment is discontinued, virus replication is rekindled. One reservoir capable of harboring HIV in a latent state and igniting renewed infection once therapy is terminated is a resting T cell. Due to the sparsity of T cells latently infected with HIV in vivo, it has been difficult to study viral and cellular interactions during latency. The SCID-hu (Thy/Liv) mouse model of HIV latency, however, provides high percentages of latently infected cells, allowing a detailed analysis of phenotype. Herein we show that latently infected cells appear phenotypically normal. Following cellular stimulation, the virus completes its life cycle and induces phenotypic changes, such as CD4 and major histocompatibility complex class I down-regulation, in the infected cell. In addition, HIV expression following activation did not correlate with expression of the cellular activation marker CD25. The apparently normal phenotype and lack of HIV expression in latently infected cells could prevent recognition by the immune response and contribute to the long-lived nature of this reservoir.
Figures





Similar articles
-
Effects of prostratin on T-cell activation and human immunodeficiency virus latency.J Virol. 2002 Aug;76(16):8118-23. doi: 10.1128/jvi.76.16.8118-8123.2002. J Virol. 2002. PMID: 12134017 Free PMC article.
-
HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model.Retrovirology. 2013 Oct 24;10:121. doi: 10.1186/1742-4690-10-121. Retrovirology. 2013. PMID: 24156277 Free PMC article.
-
HIV Replication and Latency in a Humanized NSG Mouse Model during Suppressive Oral Combinational Antiretroviral Therapy.J Virol. 2018 Mar 14;92(7):e02118-17. doi: 10.1128/JVI.02118-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343582 Free PMC article.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
-
HIV latency: experimental systems and molecular models.FEMS Microbiol Rev. 2012 May;36(3):706-16. doi: 10.1111/j.1574-6976.2012.00335.x. FEMS Microbiol Rev. 2012. PMID: 22372374 Free PMC article. Review.
Cited by
-
Regulation of HIV-1 latency by T-cell activation.Cytokine. 2007 Jul;39(1):63-74. doi: 10.1016/j.cyto.2007.05.017. Epub 2007 Jul 20. Cytokine. 2007. PMID: 17643313 Free PMC article. Review.
-
HIV latency in the humanized BLT mouse.J Virol. 2012 Jan;86(1):339-47. doi: 10.1128/JVI.06366-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072769 Free PMC article.
-
Benefits and limitations of humanized mice in HIV persistence studies.Retrovirology. 2020 Apr 6;17(1):7. doi: 10.1186/s12977-020-00516-2. Retrovirology. 2020. PMID: 32252791 Free PMC article. Review.
-
Spontaneous reactivation of latent HIV-1 promoters is linked to the cell cycle as revealed by a genetic-insulators-containing dual-fluorescence HIV-1-based vector.Sci Rep. 2018 Jul 5;8(1):10204. doi: 10.1038/s41598-018-28161-y. Sci Rep. 2018. PMID: 29977044 Free PMC article.
-
Primary cell model for activation-inducible human immunodeficiency virus.J Virol. 2007 Jul;81(14):7424-34. doi: 10.1128/JVI.02838-06. Epub 2007 May 2. J Virol. 2007. PMID: 17475639 Free PMC article.
References
-
- Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. - PubMed
-
- Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. - PubMed
-
- Blaak, H., A. B. van't Wout, M. Brouwer, B. Hooibrink, E. Hovenkamp, and H. Schuitemaker. 2000. In vivo HIV-1 infection of CD45RA(+)CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc. Natl. Acad. Sci. USA 97:1269-1274. - PMC - PubMed
-
- Borvak, J., C. S. Chou, K. Bell, G. Van Dyke, H. Zola, O. Ramilo, and E. S. Vitetta. 1995. Expression of CD25 defines peripheral blood mononuclear cells with productive versus latent HIV infection. J. Immunol. 155:3196-3204. - PubMed
-
- Brooks, D. G., S. G. Kitchen, C. M. Kitchen, D. D. Scripture-Adams, and J. A. Zack. 2001. Generation of HIV latency during thymopoiesis. Nat. Med. 7:459-464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

