Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques
- PMID: 11799168
- PMCID: PMC135900
- DOI: 10.1128/jvi.76.4.1731-1743.2002
Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques
Abstract
The ability of recombinant rhesus interleukin-12 (rMamu-IL-12) administration during acute simian immunodeficiency virus SIVmac251 infection to influence the quality of the antiviral immune responses was assessed in rhesus macaques. Group I (n = 4) was the virus-only control group. Group II and III received a conditioning regimen of rMamu-IL-12 (10 and 20 microg/kg, respectively, subcutaneously [s.c.]) on days -2 and 0. Thereafter, group II received 2 microg of IL-12 per kg and group III received 10 microg/kg s.c. twice a week for 8 weeks. On day 0 all animals were infected with SIVmac251 intravenously. While all four group I animals and three of four group II animals died by 8 and 10 months post infection (p.i.), all four group III animals remained alive for >20 months p.i. The higher IL-12 dose led to lower plasma viral loads and markedly lower peripheral blood mononuclear cell and lymph node proviral DNA loads. During the acute viremia phase, the high-IL-12-dose monkeys showed an increase in CD3(-) CD8 alpha/alpha(+) and CD3(+) CD8 alpha/alpha(+) cells and, unlike the control and low-IL-12-dose animals, did not demonstrate an increase in CD4(+) CD45RA(+) CD62L(+) naive cells. The high-IL-12-dose animals also demonstrated that both CD8 alpha/alpha(+) and CD8 alpha/beta(+) cells produced antiviral factors early p.i., whereas only CD8 alpha/beta(+) cells retained this function late p.i. Long-term survival correlated with sustained high levels of SIV gag/pol and SIV env cytotoxic T lymphocytes and retention of high memory responses against nominal antigens. This is the first study to demonstrate the capacity of IL-12 to significantly protect macaques from SIV-induced disease, and it provides a useful model to more precisely identify correlates of virus-specific disease-protective responses.
Figures


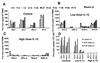
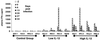
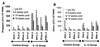

Similar articles
-
Repeated exposure of rhesus macaques to low doses of simian immunodeficiency virus (SIV) did not protect them against the consequences of a high-dose SIV challenge.J Gen Virol. 1995 Jun;76 ( Pt 6):1307-15. doi: 10.1099/0022-1317-76-6-1307. J Gen Virol. 1995. PMID: 7782761
-
Cytokine/Chemokine responses in activated CD4+ and CD8+ T cells isolated from peripheral blood, bone marrow, and axillary lymph nodes during acute simian immunodeficiency virus infection.J Virol. 2014 Aug;88(16):9442-57. doi: 10.1128/JVI.00774-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920807 Free PMC article.
-
Long-term control of simian immunodeficiency virus mac251 viremia to undetectable levels in half of infected female rhesus macaques nasally vaccinated with simian immunodeficiency virus DNA/recombinant modified vaccinia virus Ankara.J Immunol. 2011 Mar 15;186(6):3581-93. doi: 10.4049/jimmunol.1002594. Epub 2011 Feb 11. J Immunol. 2011. PMID: 21317390
-
Cannabinoid administration attenuates the progression of simian immunodeficiency virus.AIDS Res Hum Retroviruses. 2011 Jun;27(6):585-92. doi: 10.1089/aid.2010.0218. Epub 2010 Nov 23. AIDS Res Hum Retroviruses. 2011. PMID: 20874519 Free PMC article. Review.
-
The role of disease stage, plasma viral load and regulatory T cells (Tregs) on autoantibody production in SIV-infected non-human primates.J Autoimmun. 2007 Mar-May;28(2-3):152-9. doi: 10.1016/j.jaut.2007.02.014. Epub 2007 Mar 26. J Autoimmun. 2007. PMID: 17368846 Free PMC article. Review.
Cited by
-
Dendritic cell dysregulation during HIV-1 infection.Immunol Rev. 2013 Jul;254(1):170-89. doi: 10.1111/imr.12082. Immunol Rev. 2013. PMID: 23772620 Free PMC article. Review.
-
A Stronger Innate Immune Response During Hyperacute Human Immunodeficiency Virus Type 1 (HIV-1) Infection Is Associated With Acute Retroviral Syndrome.Clin Infect Dis. 2021 Sep 7;73(5):832-841. doi: 10.1093/cid/ciab139. Clin Infect Dis. 2021. PMID: 33588436 Free PMC article.
-
Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions.J Virol. 2014 Mar;88(5):2508-18. doi: 10.1128/JVI.02034-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352453 Free PMC article.
-
Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen.J Virol. 2011 Sep;85(18):9578-87. doi: 10.1128/JVI.05060-11. Epub 2011 Jul 6. J Virol. 2011. PMID: 21734035 Free PMC article.
-
Remodeling of immune system functions by extracellular vesicles.Front Immunol. 2025 Mar 13;16:1549107. doi: 10.3389/fimmu.2025.1549107. eCollection 2025. Front Immunol. 2025. PMID: 40181981 Free PMC article.
References
-
- Ahuja, S. S., R. L. Reddick, N. Sato, E. Montalbo, V. Kostecki, W. Zhao, M. J. Dolan, P. C. Melby, and S. K. Ahuja. 1999. Dendritic cell (DC)-based anti-infective strategies: DCs engineered to secrete IL-12 are a potent vaccine in a murine model of an intracellular infection. J. Immunol. 163:3890-3897. - PubMed
-
- Anonymous. 1995. Genetics Institute suspends phase II study of rhIL-12. J. Int. Assoc. Physicians AIDS Care 1:34. - PubMed
-
- Ansari, A. A., P. Bostik, A. E. Mayne, and F. Villinger. 2001. Failure to expand influenza and tetanus toxoid memory T cells in vitro correlates with disease course in SIV infected rhesus macaques. Cell. Immunol. 210:125-142. - PubMed
-
- Baier, M., A. Werner, N. Bannert, K. Metzner, and R. Kurth. 1995. HIV suppression by interleukin-16. Nature 378:563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

