The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection
- PMID: 11799183
- PMCID: PMC135906
- DOI: 10.1128/jvi.76.4.1884-1891.2002
The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection
Abstract
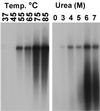
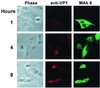
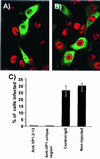
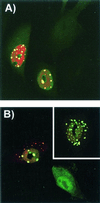
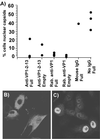
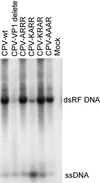
The unique N-terminal region of the parvovirus VP1 capsid protein is required for infectivity by the capsids but is not required for capsid assembly. The VP1 N terminus contains a number of groups of basic amino acids which resemble classical nuclear localization sequences, including a conserved sequence near the N terminus comprised of four basic amino acids, which in a peptide can act to transport other proteins into the cell nucleus. Testing with a monoclonal antibody recognizing residues 2 to 13 of VP1 (anti-VP1-2-13) and with a rabbit polyclonal serum against the entire VP1 unique region showed that the VP1 unique region was not exposed on purified capsids but that it became exposed after treatment of the capsids with heat (55 to 75 degrees C), or urea (3 to 5 M). A high concentration of anti-VP1-2-13 neutralized canine parvovirus (CPV) when it was incubated with the virus prior to inoculation of cells. Both antibodies blocked infection when injected into cells prior to virus inoculation, but neither prevented infection by coinjected infectious plasmid DNA. The VP1 unique region could be detected 4 and 8 h after the virus capsids were injected into cells, and that sequence exposure appeared to be correlated with nuclear transport of the capsids. To examine the role of the VP1 N terminus in infection, we altered that sequence in CPV, and some of those changes made the capsids inefficient at cell infection.
Figures
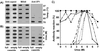
References
-
- Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. - PubMed
-
- Anderson, S., M. Momoeda, M. Kawase, S. Kajigaya, and N. S. Young. 1995. Peptides derived from the unique region of B19 parvovirus minor capsid protein elicit neutralizing antibodies in rabbits. Virology 206:626-632. - PubMed
-
- Casal, J. I., J. P. Langeveld, E. Cortes, W. W. Schaaper, E. van Dijk, C. Vela, S. Kamstrup, and R. H. Meloen. 1995. Peptide vaccine against canine parvovirus: identification of two neutralization subsites in the N terminus of VP2 and optimization of the amino acid sequence. J. Virol. 69:7274-7277. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

