The VP1 unique region of parvovirus B19 and its constituent phospholipase A2-like activity
- PMID: 11799199
- PMCID: PMC135890
- DOI: 10.1128/jvi.76.4.2014-2018.2002
The VP1 unique region of parvovirus B19 and its constituent phospholipase A2-like activity
Abstract
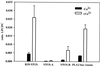
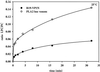
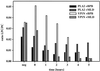
Parvovirus B19 is the causative agent of erythema infectiosum. In addition, parvovirus B19 infection may be associated with other disease manifestations, namely, thrombocytopenia or granulocytopenia, spontaneous abortion or hydrops fetalis in pregnant women, acute and chronic arthritis, and systemic lupus erythematosus. Based on sequence homology data, a phospholipase A2 motif has been identified in the VP1 unique region of parvovirus B19. (Y. Li et al., J. Gen. Virol. 82:2821-2825, 2001; Z. Zadori et al., Dev. Cell 1:291-302, 2001). We have established a new in vitro assay based on electrospray ionization tandem mass spectroscopy to show that phospholipase A2 activity is present in the VP1 unique region produced in Escherichia coli and in virus-like particles consisting of combinations of VP1 and VP2 proteins expressed by recombinant baculovirus. The enzyme activity of the VP1 unique region showed typical Ca(2+) dependency and could be inhibited by manoalide and 4-bromophenacylbromide, which bind covalently to lysine and histidine residues, respectively, as part of the active center of the enzyme. By using subfragments, we demonstrated an association between the phospholipase A2-like activity and the carboxy-terminal domain of the VP1 unique region.
Figures


References
-
- Arni, R. K., and R. J. Ward. 1996. Phospholipase A2—a structural review. Toxicon 34:827-841. - PubMed
-
- Bekkers, A. C., P. A. Franken, E. Toxopeus, H. M. Verheij, and G. H. de Haas. 1991. The importance of glycine-30 for enzymatic activity of phospholipase A2. Biochim. Biophys. Acta 1076:374-378. - PubMed
-
- Bligh, E. G., and W. J. Dyer. A rapid method of total lipid extraction and purification. 1956. Can. J. Biochem. Physiol. 37:911-917. - PubMed
-
- Brown, K. E., S. M. Anderson, and N. S. Young. 1993. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114-117. - PubMed
-
- Brown, T., A. Anand, L. D. Ritchie, J. P. Clewley, and T. M. Reid. 1984. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet ii:1033-1034. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

