Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition
- PMID: 11801722
- PMCID: PMC2933194
- DOI: 10.1242/jcs.115.1.39
Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition
Abstract
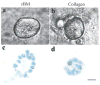
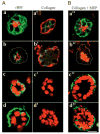
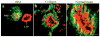
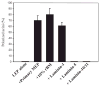
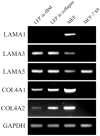
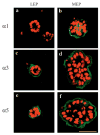
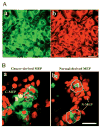
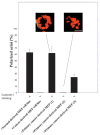
The signals that determine the correct polarity of breast epithelial structures in vivo are not understood. We have shown previously that luminal epithelial cells can be polarized when cultured within a reconstituted basement membrane gel. We reasoned that such cues in vivo may be given by myoepithelial cells. Accordingly, we used an assay where luminal epithelial cells are incorrectly polarized to test this hypothesis. We show that culturing human primary luminal epithelial cells within collagen-I gels leads to formation of structures with no lumina and with reverse polarity as judged by dual stainings for sialomucin, epithelial specific antigen or occludin. No basement membrane is deposited, and beta4-integrin staining is negative. Addition of purified human myoepithelial cells isolated from normal glands corrects the inverse polarity, and leads to formation of double-layered acini with central lumina. Among the laminins present in the human breast basement membrane (laminin-1, -5 and -10/11), laminin-1 was unique in its ability to substitute for myoepithelial cells in polarity reversal. Myoepithelial cells were purified also from four different breast cancer sources including a biphasic cell line. Three out of four samples either totally lacked the ability to interact with luminal epithelial cells, or conveyed only correction of polarity in a fraction of acini. This behavior was directly related to the ability of the tumor myoepithelial cells to produce alpha-1 chain of laminin. In vivo, breast carcinomas were either negative for laminin-1 (7/12 biopsies) or showed a focal, fragmented deposition of a less intensely stained basement membrane (5/12 biopsies). Dual staining with myoepithelial markers revealed that tumor-associated myoepithelial cells were either negative or weakly positive for expression of laminin-1, establishing a strong correlation between loss of laminin-1 and breast cancer. We conclude that the double-layered breast acinus may be recapitulated in culture and that one reason for the ability of myoepithelial cells to induce polarity is because they are the only source of laminin-1 in the breast in vivo. A further conclusion is that a majority of tumor-derived/-associated myoepithelial cells are deficient in their ability to impart polarity because they have lost their ability to synthesize sufficient or functional laminin-1. These results have important implications for the role of myoepithelial cells in maintenance of polarity in normal breast and how they may function as structural tumor suppressors.
Figures


References
-
- Albrechtsen R, Nielsen M, Wewer U, Engvall E, Rouslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981;41:5076–5081. - PubMed
-
- Briand P, Lykkesfeldt A. Long-term cultivation of a human breast cancer cell line, MCF-7, in a chemically defined medium. Effect of estradiol. Anticancer Res. 1986;6:85–90. - PubMed
-
- Clarke C, Titley J, Davies S, O’Hare MJ. An immunomagnetic separation method using superparamagnetic (MACS) beads for large-scale purification of human mammary luminal and myoepithelial cells. Epithelial Cell Biol. 1994;3:38–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

