Complement activation by recombinant adenoviruses
- PMID: 11803399
- PMCID: PMC7091591
- DOI: 10.1038/sj.gt.3301611
Complement activation by recombinant adenoviruses
Abstract
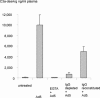
Recombinant adenoviruses are currently the most important vector system in gene therapy. Adenoviruses frequently cause upper respiratory tract infections in humans and anti-adenoviral antibodies are found in 35-70% of the population. Therefore in the majority of potential patients receiving adenoviral gene therapy, the contact of virus particles and blood will lead to the formation of antigen-antibody complexes. These complexes have the ability to induce inflammatory reactions via an activation of the complement system. We have determined the level of C3a (the most reactive complement component) generated in isolated citrate plasma of healthy individuals after challenge with recombinant and wild-type adenoviruses in amounts corresponding to virus blood levels to be expected in patients during adenoviral gene therapy. All plasma samples containing anti-adenoviral antibodies showed a substantial, dose-dependent generation of C3a. A virus plasma level of about 7.5 x 10(9) particles/ml (which was calculated to be the highest blood level reached during clinical trials in the past) induced an average release of about 3000 ng/ml C3a (baseline levels <140 ng/ml). Analyzing the nature of anti-adenoviral antibodies showed, that not only antibodies with neutralizing properties (anti-Ad5), but also non-neutralizing anti-adenoviral antibodies are capable of complement activation. This study suggests that complement activation can be ignored in local low-dose applications of recombinant adenoviruses, but warrants attention after systemic application of large viral quantities. In clinical protocols aiming at systemic virus application, measures for monitoring and controlling the complement system should be included on a regular basis.
Figures


References
-
- Batshaw ML et al. Recombinant adenovirus gene transfer in adults with partial ornithine transcarbamylase deficiency (OTCD) - PubMed
-
- Volankis JE, Frank MM. The Human Complement System in Health and Disease. 1998.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

