Beta2 integrins are required for skin homing of primed T cells but not for priming naive T cells
- PMID: 11805130
- PMCID: PMC150832
- DOI: 10.1172/JCI11703
Beta2 integrins are required for skin homing of primed T cells but not for priming naive T cells
Abstract
Beta2 integrins are of critical importance for leukocyte extravasation through vascular endothelia and for T cell activation. To elucidate the role of beta2 integrins in T cell-mediated immune responses, allergic contact dermatitis (ACD), irritant dermatitis, and delayed-type hypersensitivity (DTH) were assessed in mice lacking the beta2 integrin subunit, CD18. ACD and DTH responses, but not edema formation, were severely suppressed in CD18(-/-) mice. Extravasation of CD18(-/-) T cells into eczematous skin lesions was greatly impaired, whereas migration of Langerhans cell precursors and dendritic cells was normal in CD18(-/-) mice. CD18(-/-)lymph nodes (LNs) contained an abnormal population of CD3(-)CD44(high) lymphocytes and showed evidence of widespread T cell activation. T cells from regional LNs of sensitized CD18(-/-) mice proliferated in response to hapten challenge, and subcutaneous injection of sensitized syngeneic LN cells directly into ears of hapten-challenged naive recipients restored the defective ACD in CD18(-/-) mice, suggesting that CD18 is not required for priming of naive T cells but is indispensable for T cell extravasation. Thus, a dysfunction of T cells, in addition to granulocytes, may contribute to the pathophysiology of leukocyte adhesion deficiency type I, which arises from mutations in the human CD18 gene.
Figures
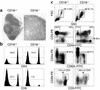
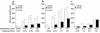

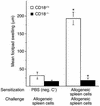

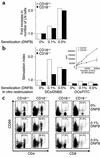


References
-
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. - PubMed
-
- Issekutz TB. Lymphocyte homing to sites of inflammation. Curr Opin Immunol. 1992;4:287–293. - PubMed
-
- Luscinskas FW, Lawler J. Integrins as dynamic regulators of vascular function. FASEB J. 1994;8:929–938. - PubMed
-
- Benimetskaya L, et al. Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med. 1997;4:414–420. - PubMed
-
- El Ghmati SM, van Hoeyveld EM, van Strijp JAG, Ceuppens JL, Stevens EAM. Identification of haptoglobin as an alternative ligand for CD11b/CD18. J Immunol. 1996;156:2542–2552. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

