Normal Th1 development following long-term therapeutic blockade of CD154-CD40 in experimental autoimmune encephalomyelitis
- PMID: 11805135
- PMCID: PMC150843
- DOI: 10.1172/JCI14374
Normal Th1 development following long-term therapeutic blockade of CD154-CD40 in experimental autoimmune encephalomyelitis
Abstract
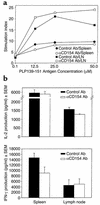
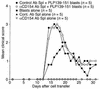
Experimental autoimmune encephalomyelitis (EAE) is a Th1-mediated demyelinating disease of the CNS with similarities to multiple sclerosis. We and others have shown that a short-term course of anti-CD154 mAb treatment to block CD154-CD40 interactions can be used to prevent or even treat ongoing PLP139-151-induced relapsing EAE. However, little is known of the long-term effects of CD154 blockade on the development of antigen-specific T cell function. Here, we show that short-term treatment with anti-CD154 at the time of PLP139-151/CFA immunization inhibits clinical disease for up to 100 days after immunization. At this point, comparable numbers of Th1 cells are observed in anti-CD154 and control Ig-treated mice, as assessed by antigen-specific ELISPOT assays. Thus, the long-term Th1/Th2 balance is largely unaffected. Inflammatory responses are diminished in anti-CD154-treated mice, as indicated by reduced in vivo delayed-type hypersensitivity and reduced levels of splenic IFN-gamma secretion in vitro. However, upon adoptive transfer of T cells isolated from the spleens of anti-CD154-treated mice, these cells contributed as effectively to clinical disease as those obtained from control-treated mice. Thus, anti-CD154 therapy leads to long-term therapeutic efficacy without exerting a long-term influence on Th1 development.
Figures


Similar articles
-
Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis.J Clin Invest. 1999 Jan;103(2):281-90. doi: 10.1172/JCI5388. J Clin Invest. 1999. PMID: 9916140 Free PMC article.
-
Autoimmune intervention by CD154 blockade prevents T cell retention and effector function in the target organ.J Immunol. 2001 Feb 1;166(3):1547-53. doi: 10.4049/jimmunol.166.3.1547. J Immunol. 2001. PMID: 11160195
-
FcR interactions do not play a major role in inhibition of experimental autoimmune encephalomyelitis by anti-CD154 monoclonal antibodies.J Immunol. 2004 Jul 15;173(2):993-9. doi: 10.4049/jimmunol.173.2.993. J Immunol. 2004. PMID: 15240687
-
Transient anti-CD154-mediated immunotherapy of ongoing relapsing experimental autoimmune encephalomyelitis induces long-term inhibition of disease relapses.J Neuroimmunol. 2002 Aug;129(1-2):58-65. doi: 10.1016/s0165-5728(02)00175-3. J Neuroimmunol. 2002. PMID: 12161021
-
Characteristics of the T lymphocytes involved in experimental allergic encephalomyelitis.J Neuroimmunol. 1995 Sep;61(2):107-16. doi: 10.1016/0165-5728(95)00109-f. J Neuroimmunol. 1995. PMID: 7593546 Review.
Cited by
-
CD40 Drives Central Nervous System Autoimmune Disease by Inducing Complementary Effector Programs via B Cells and Dendritic Cells.J Immunol. 2022 Dec 1;209(11):2083-2092. doi: 10.4049/jimmunol.2200439. J Immunol. 2022. PMID: 36426970 Free PMC article.
-
Regulation and function of class II major histocompatibility complex, CD40, and B7 expression in macrophages and microglia: Implications in neurological diseases.J Neurovirol. 2002 Dec;8(6):496-512. doi: 10.1080/13550280290100941. J Neurovirol. 2002. PMID: 12476345 Review.
-
PPE57 induces activation of macrophages and drives Th1-type immune responses through TLR2.J Mol Med (Berl). 2015 Jun;93(6):645-62. doi: 10.1007/s00109-014-1243-1. Epub 2015 Jan 15. J Mol Med (Berl). 2015. PMID: 25586105
-
The CD40-CD40L Dyad in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis.Front Immunol. 2017 Dec 12;8:1791. doi: 10.3389/fimmu.2017.01791. eCollection 2017. Front Immunol. 2017. PMID: 29312317 Free PMC article. Review.
-
Soluble mannosylated myelin peptide inhibits the encephalitogenicity of autoreactive T cells during experimental autoimmune encephalomyelitis.Am J Pathol. 2007 Jan;170(1):272-80. doi: 10.2353/ajpath.2007.060335. Am J Pathol. 2007. PMID: 17200200 Free PMC article.
References
-
- Brown AM, McFarlin DE. Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest. 1981;45:278–284. - PubMed
-
- McRae BL, Kennedy MK, Tan LJ, Dal Canto MC, Miller SD. Induction of active and adoptive chronic-relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J Neuroimmunol. 1992;38:229–240. - PubMed
-
- Olson JK, Croxford JL, Miller SD. Virus-induced autoimmunity: potential role of viruses in initiation, perpetuation, and progression of T cell-mediated autoimmune diseases. Viral Immunol. 2001;14:227–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

