Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation
- PMID: 11805146
- PMCID: PMC2193613
- DOI: 10.1084/jem.20011571
Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation
Abstract
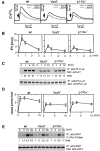
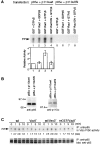
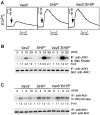
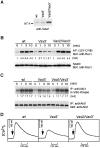
To elucidate the mechanism(s) by which Vav3, a new member of the Vav family proteins, participates in B cell antigen receptor (BCR) signaling, we have generated a B cell line deficient in Vav3. Here we report that Vav3 influences phosphoinositide 3-kinase (PI3K) function through Rac1 in that phosphatidylinositol-3,4,5-trisphosphate (PIP3) generation was attenuated by loss of Vav3 or by expression of a dominant negative form of Rac1. The functional interaction between PI3K and Rac1 was also demonstrated by increased PI3K activity in the presence of GTP-bound Rac1. In addition, we show that defects of calcium mobilization and c-Jun NH2-terminal kinase (JNK) activation in Vav3-deficient cells are relieved by deletion of a PIP3 hydrolyzing enzyme, SH2 domain-containing inositol polyphosphate 5'-phosphatase (SHIP). Hence, our results suggest a role for Vav3 in regulating the B cell responses by promoting the sustained production of PIP3 and thereby calcium flux.
Figures






Similar articles
-
Distinct role of phosphatidylinositol 3-kinase and Rho family GTPases in Vav3-induced cell transformation, cell motility, and morphological changes.J Biol Chem. 2002 May 17;277(20):17638-48. doi: 10.1074/jbc.M111575200. Epub 2002 Mar 7. J Biol Chem. 2002. PMID: 11884391
-
Activation of Rac1 and the exchange factor Vav3 are involved in NPM-ALK signaling in anaplastic large cell lymphomas.Oncogene. 2008 Apr 24;27(19):2728-36. doi: 10.1038/sj.onc.1210921. Epub 2007 Nov 12. Oncogene. 2008. PMID: 17998938
-
Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells.Mol Biol Cell. 2005 May;16(5):2207-17. doi: 10.1091/mbc.e04-10-0904. Epub 2005 Feb 23. Mol Biol Cell. 2005. PMID: 15728722 Free PMC article.
-
B-cell development and antigen receptor signalling.Biochem Soc Trans. 2002 Aug;30(4):812-5. doi: 10.1042/bst0300812. Biochem Soc Trans. 2002. PMID: 12196204 Review.
-
Regulation of BCR signaling.Mol Immunol. 2011 Jun;48(11):1287-91. doi: 10.1016/j.molimm.2010.12.007. Epub 2010 Dec 31. Mol Immunol. 2011. PMID: 21195477 Review.
Cited by
-
Phospholipase C-gamma2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen.J Exp Med. 2008 Apr 14;205(4):853-68. doi: 10.1084/jem.20072619. Epub 2008 Mar 24. J Exp Med. 2008. PMID: 18362175 Free PMC article.
-
Nonobese diabetic congenic strain analysis of autoimmune diabetes reveals genetic complexity of the Idd18 locus and identifies Vav3 as a candidate gene.J Immunol. 2010 May 1;184(9):5075-84. doi: 10.4049/jimmunol.0903734. Epub 2010 Apr 2. J Immunol. 2010. PMID: 20363978 Free PMC article.
-
The Function of Embryonic Stem Cell-expressed RAS (E-RAS), a Unique RAS Family Member, Correlates with Its Additional Motifs and Its Structural Properties.J Biol Chem. 2015 Jun 19;290(25):15892-15903. doi: 10.1074/jbc.M115.640607. Epub 2015 May 4. J Biol Chem. 2015. PMID: 25940089 Free PMC article.
-
BTK inhibitors in the treatment of hematological malignancies and inflammatory diseases: mechanisms and clinical studies.J Hematol Oncol. 2022 Oct 1;15(1):138. doi: 10.1186/s13045-022-01353-w. J Hematol Oncol. 2022. PMID: 36183125 Free PMC article. Review.
-
The Vav family: at the crossroads of signaling pathways.Immunol Res. 2005;32(1-3):259-65. doi: 10.1385/IR:32:1-3:259. Immunol Res. 2005. PMID: 16106078 Review.
References
-
- DeFranco, A.L. 1997. The complexity of signaling pathways activated by the BCR. Curr. Opin. Immunol. 9:296–308. - PubMed
-
- Reth, M., and J. Wienands. 1997. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15:453–479. - PubMed
-
- Tamir, I., and J.C. Cambier. 1998. Antigen receptor signaling: integration of protein tyrosine kinase functions. Oncogene. 17:1353–1364. - PubMed
-
- Kurosaki, T. 1999. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17:555–592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

