A novel member of the leukocyte receptor complex regulates osteoclast differentiation
- PMID: 11805147
- PMCID: PMC2193610
- DOI: 10.1084/jem.20011681
A novel member of the leukocyte receptor complex regulates osteoclast differentiation
Abstract
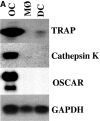
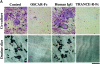
Osteoclasts (OCs) are multinucleated cells that resorb bone and are essential for bone homeostasis. They develop from hematopoietic cells of the myelomonocytic lineage. OC formation requires cell-to-cell interactions with osteoblasts and can be achieved by coculturing bone marrow precursor cells with osteoblasts/stromal cells. Two of the key factors mediating the osteoblast-induced osteoclastogenesis are macrophage-colony stimulating factor (M-CSF) and the tumor necrosis factor (TNF) family member TNF-related activation-induced cytokine (TRANCE) that are produced by osteoblasts/stromal cells in response to various bone resorbing hormones. In addition, other factors produced by osteoblasts/stromal cells further influence osteoclastogenesis. Here we report the identification and characterization of OC-associated receptor (OSCAR), a novel member of the leukocyte receptor complex (LRC)-encoded family expressed specifically in OCs. Genes in the LRC produce immunoglobulin (Ig)-like surface receptors and play critical roles in the regulation of both innate and adaptive immune responses. Different from the previously characterized members of the LRC complex, OSCAR expression is detected specifically in preosteoclasts or mature OCs. Its putative-ligand (OSCAR-L) is expressed primarily in osteoblasts/stromal cells. Moreover, addition of a soluble form of OSCAR in coculture with osteoblasts inhibits the formation of OCs from bone marrow precursor cells in the presence of bone-resorbing factors, indicating that OSCAR may be an important bone-specific regulator of OC differentiation. In addition, this study suggests that LRC-encoded genes may have evolved to regulate the physiology of cells beyond those of the immune system.
Figures










References
-
- Suda, T., N. Takahashi, N. Udagawa, E. Jimi, M.T. Gillespie, and T.J. Martin. 1999. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20:345–357. - PubMed
-
- Teitelbaum, S.L. 2000. Bone resorption by osteoclasts. Science. 289:1504–1508. - PubMed
-
- Yoshida, H., S. Hayashi, T. Kunisada, M. Ogawa, S. Nishikawa, H. Okamura, T. Sudo, and L.D. Shultz. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 345:442–444. - PubMed
-
- Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, S. Mochizuki, A. Tomoyasu, K. Yano, M. Goto, A. Murakami, et al. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA. 95:3597–3602. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

