Complement C3 activation is required for antiphospholipid antibody-induced fetal loss
- PMID: 11805148
- PMCID: PMC2193604
- DOI: 10.1084/jem.200116116
Complement C3 activation is required for antiphospholipid antibody-induced fetal loss
Abstract
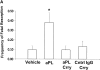
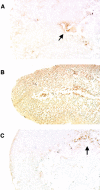
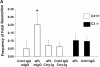
The antiphospholipid syndrome (APS) is characterized by recurrent fetal loss, vascular thrombosis, and thrombocytopenia occurring in the presence of antiphospholipid (aPL) antibodies. The pathogenesis of fetal loss and tissue injury in APS is incompletely understood, but is thought to involve platelet and endothelial cell activation as well as procoagulant effects of aPL antibodies acting directly on clotting pathway components. Recent studies have shown that uncontrolled complement activation in the placenta leads to fetal death in utero. We hypothesized that aPL antibodies activate complement in the placenta, generating split products that mediate placental injury and lead to fetal loss and growth retardation. To test this hypothesis, we used a murine model of APS in which pregnant mice are injected with human IgG containing aPL antibodies. We found that inhibition of the complement cascade in vivo, using the C3 convertase inhibitor complement receptor 1-related gene/protein y (Crry)-Ig, blocks fetal loss and growth retardation. Furthermore, mice deficient in complement C3 were resistant to fetal injury induced by aPL antibodies. While antigenic epitopes recognized by aPL antibodies are important in the pathogenesis of APS, our data show that in vivo complement activation is required for aPL antibody-induced fetal loss and growth retardation.
Figures




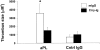
References
-
- Branch, D.W., D.J. Dudley, M.D. Mitchell, K.A. Creighton, T.M. Abbott, E.H. Hammond, and R.A. Daynes. 1990. Immunoglobulin G fractions from patients with antiphospholipid antibodies cause fetal death in BALB/c mice: a model for autoimmune fetal loss. Am. J. Obstet. Gynecol. 163:210–216. - PubMed
-
- Piona, A., L. La Rosa, A. Tincani, D. Faden, G. Magro, S. Grasso, F. Nicoletti, G. Balestrieri, and P.L. Meroni. 1995. Placental thrombosis and fetal loss after passive transfer of mouse lupus monoclonal or human polyclonal anti-cardiolipin antibodies in pregnant naive BALB/c mice. Scand. J. Immunol. 41:427–432. - PubMed
-
- Ikematsu, W., F.L. Luan, L. La Rosa, B. Beltrami, F. Nicoletti, J.P. Buyon, P.L. Meroni, G. Balestrieri, and P. Casali. 1998. Human anticardiolipin monoclonal autoantibodies cause placental necrosis and fetal loss in BALB/c mice. Arthr. Rheum. 41:1026–1039. - PubMed
-
- Di Simone, N., P.L. Meroni, N. Del Papa, E. Raschi, D. Caliandro, S. del Carlolis, M.A. Khamashta, T. Atsuni, G.R.V. Hughes, G. Balestrieri, et al. 2000. Antiphospholipid antibodies affect trophoblast gonadotropin secretion and invasiveness by binding directly and through adhered B2-glycoprotein 1. Arthr. Rheum. 43:140–150. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

