Requirement for the NF-kappaB family member RelA in the development of secondary lymphoid organs
- PMID: 11805150
- PMCID: PMC2193608
- DOI: 10.1084/jem.20011885
Requirement for the NF-kappaB family member RelA in the development of secondary lymphoid organs
Abstract
The transcription factor nuclear factor (NF)-kappaB has been suggested to be a key mediator of the development of lymph nodes and Peyer's patches. However, targeted deletion of NF-kappaB/ Rel family members has not yet corroborated such a function. Here we report that when mice lacking the RelA subunit of NF-kappaB are brought to term by breeding onto a tumor necrosis factor receptor (TNFR)1-deficient background, the mice that are born lack lymph nodes, Peyer's patches, and an organized splenic microarchitecture, and have a profound defect in T cell-dependent antigen responses. Analyses of TNFR1/RelA-deficient embryonic tissues and of radiation chimeras suggest that the dependence on RelA is manifest not in hematopoietic cells but rather in radioresistant stromal cells needed for the development of secondary lymphoid organs.
Figures

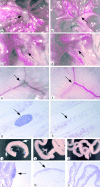
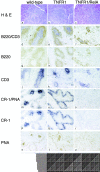
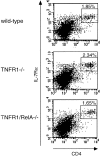

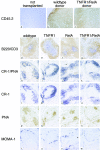
References
-
- Karrer, U., A. Althage, B. Odermatt, C.W. Roberts, S.J. Korsmeyer, S. Miyawaki, H. Hengartner, and R.M. Zinkernagel. 1997. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(−)/−) mutant mice. J. Exp. Med. 185:2157–2170. - PMC - PubMed
-
- Shinkura, R., K. Kitada, F. Matsuda, K. Tashiro, K. Ikuta, M. Suzuki, K. Kogishi, T. Serikawa, and T. Honjo. 1999. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat. Genet. 22:74–77. - PubMed
-
- Kong, Y.Y., H. Yoshida, I. Sarosi, H.L. Tan, E. Timms, C. Capparelli, S. Morony, A.J. Oliveira-dos-Santos, G. Van, A. Itie, et al. 1999. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 397:315–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

