Subnuclear distribution of topoisomerase I is linked to ongoing transcription and p53 status
- PMID: 11805286
- PMCID: PMC122173
- DOI: 10.1073/pnas.022631899
Subnuclear distribution of topoisomerase I is linked to ongoing transcription and p53 status
Erratum in
- Proc Natl Acad Sci U S A 2002 Mar 19;99(6):4134
Abstract
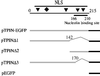
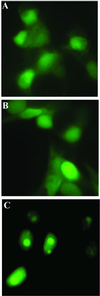
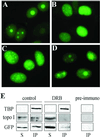
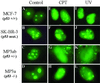
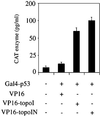
The nonconserved, hydrophilic N-terminal domain of eukaryotic DNA topoisomerase I (topo I) is dispensable for catalytic activity in vitro but essential in vivo. There are at least five putative nuclear localization signals and a nucleolin-binding signal within the first 215 residues of the topo I N-terminal domain. We have investigated physiological functions of the topo I N-terminal domain by fusing it to an enhanced green fluorescent protein (EGFP). The first 170 residues of the N-terminal domain allow efficient import of chimeric proteins into nuclei and nucleoli. The nucleolar localization of this protein does not depend on its interaction with nucleolin, whereas ongoing rDNA transcription clearly is crucial. Immunoprecipitation experiments reveal that the topo I N terminus (topoIN)-EGFP fusion protein associates with the TATA-binding protein in cells. Furthermore, DNA damage results in extensive nuclear redistribution of the topoIN-EGFP chimeric product. The redistribution is also p53-dependent and the N terminus of topo I appears to interact with p53 in vivo. These results show that the topo I localization to the nucleolus is related to the p53 and DNA damage, as well as changes in transcriptional status. Nucleolar release of topo I under conditions of cellular duress may represent an important, antecedent step in tumor cell killing by topoisomerase active agents.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

