mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties
- PMID: 11805321
- PMCID: PMC117358
- DOI: 10.1073/pnas.022637199
mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties
Abstract
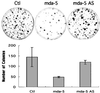
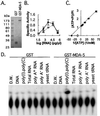
Human melanoma cells can be reprogrammed to terminally differentiate and irreversibly lose proliferative capacity by appropriate pharmacological manipulation. Subtraction hybridization identified melanoma differentiation-associated gene-5 (mda-5) as a gene induced during differentiation, cancer reversion, and programmed cell death (apoptosis). This gene contains both a caspase recruitment domain and putative DExH group RNA helicase domains. Atypical helicase motifs of MDA-5 deviate from consensus sequences but are well conserved in a potentially new group of cloned and hypothetical proteins. mda-5 is an early response gene inducible by IFN and tumor necrosis factor-alpha, responding predominantly to IFN-beta. Protein kinase C activation by mezerein further augments mda-5 expression induced by IFN-beta. Expression of mda-5 is controlled transcriptionally by IFN-beta, and the MDA-5 protein localizes in the cytoplasm. mda-5 displays RNA-dependent ATPase activity, and ectopic expression of mda-5 in human melanoma cells inhibits colony formation. In these contexts, mda-5 may function as a mediator of IFN-induced growth inhibition and/or apoptosis. MDA-5 is a double-stranded RNA-dependent ATPase that contains both a caspase recruitment domain and RNA helicase motifs, with a confirmed association with growth and differentiation in human melanoma cells.
Figures

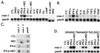

References
-
- Leszczyniecka M, Robert T, Dent P, Grant S, Fisher P B. Pharmacol Ther. 2001;90:105–156. - PubMed
-
- Waxman S. Differentiation Therapy. Vol. 10. Rome: Ares-Serono Symposia Publishers; 1996. pp. 1–531.
-
- Freemerman A J, Vrana J A, Tombes R M, Jiang H, Chellappan S P, Fisher P B, Grant S. Leukemia. 1997;11:504–513. - PubMed
-
- Jiang H, Lin J, Fisher P B. Mol Cell Differ. 1994;2:221–239.
-
- Wang Z, Su Z Z, Fisher P B, Wang S, VanTuyle G, Grant S. Exp Cell Res. 1998;244:105–116. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

