Reactive oxygen generated by Nox1 triggers the angiogenic switch
- PMID: 11805326
- PMCID: PMC117371
- DOI: 10.1073/pnas.022630199
Reactive oxygen generated by Nox1 triggers the angiogenic switch
Abstract
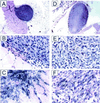
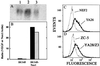
The reactive oxygen-generating enzyme Nox1 transforms NIH 3T3 cells, rendering them highly tumorigenic and, as shown herein, also increases tumorigenicity of DU-145 prostate epithelial cells. Although Nox1 modestly stimulates cell division in both fibroblasts and epithelial cells, an increased mitogenic rate alone did not account fully for the marked tumorigenicity. Herein, we show that Nox1 is a potent trigger of the angiogenic switch, increasing the vascularity of tumors and inducing molecular markers of angiogenesis. Vascular endothelial growth factor (VEGF) mRNA becomes markedly up-regulated by Nox1 both in cultured cells and in tumors, and VEGF receptors (VEGFR1 and VEGFR2) are highly induced in vascular cells in Nox1-expressing tumors. Matrix metalloproteinase activity, another marker of the angiogenic switch, also is induced by Nox1. Nox1 induction of VEGF is eliminated by coexpression of catalase, indicating that hydrogen peroxide signals part of the switch to the angiogenic phenotype.
Figures




References
-
- Suh Y-A, Arnold R S, Lassegue B, Shi J, Xu X, Sorescu D, Chung A B, Griendling K K, Lambeth J D. Nature (London) 1999;401:79–82. - PubMed
-
- Pentland A P, Morrison A R, Jacobs S C, Hruza L L, Hebert J S, Packer L. J Biol Chem. 1992;267:15578–15584. - PubMed
-
- Nakahara H, Kanno T, Inai Y, Utsumi K, Hiramatsu M, Mori A, Packer L. Free Radical Biol Med. 1998;24:85–92. - PubMed
-
- Dang C V, Semenza G L. Trends Biochem Sci. 1999;24:68–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

