Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome
- PMID: 11805328
- PMCID: PMC117376
- DOI: 10.1073/pnas.012585199
Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome
Abstract
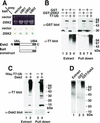
Dsk2p from Saccharomyces cerevisiae belongs to the class of proteins that contain a ubiquitin-like (UbL) domain at the N terminus together with a ubiquitin-associated (UBA) domain at the C terminus. We show here that the C-terminal UBA domain of Dsk2p binds to K48-linked polyubiquitin chains, and the N-terminal UbL domain of Dsk2p interacts with the proteasome. Overexpression of Dsk2p caused the accumulation of large amounts of polyubiquitin, and extragenic suppressors of the Dsk2p-mediated lethality proved to be temperature-sensitive mutations in two proteasome subunits, rpn1 and pre2. K48-linked ubiquitin-dependent degradation was impaired by disruption of the DSK2 gene. These results indicate that Dsk2p is K48-linked polyubiquitin-binding protein and also interacts with the proteasome. We discuss a possible role of adaptor function of Dsk2p via its UbL and UBA domains in the ubiquitin-proteasome pathway.
Figures


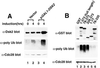


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

