Actin cable dynamics in budding yeast
- PMID: 11805329
- PMCID: PMC117377
- DOI: 10.1073/pnas.022462899
Actin cable dynamics in budding yeast
Abstract
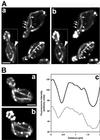
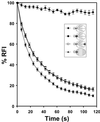
Actin cables, bundles of actin filaments that align along the long axis of budding yeast, are crucial for establishment of cell polarity. We fused green fluorescent protein (GFP) to actin binding protein 140 (Abp140p) and visualized actin cable dynamics in living yeast. We detected two populations of actin cables: (i) bud-associated cables, which extend from the bud along the mother-bud axis, and (ii) randomly oriented cables, which are relatively short. Time-lapse imaging of Abp140p-GFP revealed an apparent increase in the length of bud-associated actin cables. Analysis of movement of Abp140p-GFP fiduciary marks on bud-associated cables and fluorescence loss in photobleaching experiments revealed that this apparent elongation occurs by assembly of new material at the end of the cable within the bud and movement of the opposite end of the cable toward the tip of the mother cell distal to the bud. The rate of extension of the tip of an elongating actin cable is 0.29 +/- 0.08 microm/s. Latrunculin A (Lat-A) treatment completely blocked this process. We also observed movement of randomly oriented cables around the cortex of cells at a rate of 0.59 +/- 0.14 microm/s. Mild treatment with Lat-A did not affect the velocity of movement of randomly oriented cables. However, Lat-A treatment did increase the number of randomly oriented, motile cables per cell. Our observations suggest that establishment of bud-associated actin cables during the cell cycle is accomplished not by realignment of existing cables but by assembly of new cables within the bud or bud neck, followed by elongation.
Figures




References
-
- Cheney R E, O'Shea M K, Heuser J E, Coelho M V, Wolenski J S, Espreafico E M, Forscher P, Larson R E, Mooseker M S. Cell. 1993;75:13–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

