Design and intermediate results of the Lower Extremity Arterial Disease Event Reduction (LEADER)* trial of bezafibrate in men with lower extremity arterial disease [ISRCTN4119421]
- PMID: 11806795
- PMCID: PMC57751
- DOI: 10.1186/cvm-2-4-195
Design and intermediate results of the Lower Extremity Arterial Disease Event Reduction (LEADER)* trial of bezafibrate in men with lower extremity arterial disease [ISRCTN4119421]
Abstract
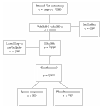
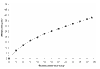
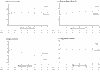
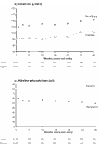
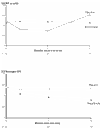
BACKGROUND: Raised levels of both triglycerides and fibrinogen, each of which are reduced by bezafibrate, may contribute to lower extremity arterial disease (LEAD). This condition is characterized by a particularly high incidence of coronary heart disease (CHD) and stroke, but is little studied thus far in randomised controlled trials. METHOD: Patients were recruited through 85 practices in the British Medical Research Council General Practice Research Framework and through nine hospital vascular clinics. The treatment regimen, which is double-blind and placebo-controlled, is bezafibrate 400 mg/day. The 1568 patients recruited represent 86% of those eligible at screening. RESULTS: None of the anticipated side effects (mainly gastrointestinal) differed between the two groups. Nearly 80% of the total person-years accrued at 3 years were spent on trial treatment. Bezafibrate significantly reduced total cholesterol by approximately 8.0% and low-density lipoprotein (LDL)-cholesterol by approximately 9.0%, and increased high-density lipoprotein (HDL)-cholesterol by approximately 11.0% initially, falling to about 6.0% at 3 years. Triglycerides were significantly reduced by about 23.0% and fibrinogen by about 14.0%. Plasma creatinine rose by approximately 11% in those on active treatment. All of these effects were highly significant (P < 0.0001). Bezafibrate had no effect on the level of C-reactive protein (CRP). CONCLUSION: The trial recruited an unusually high proportion of eligible patients, ensuring the general applicability of its results. The fibrinogen-lowering and lipid-modifying effects of bezafibrate were confirmed. Although bezafibrate lowers fibrinogen, it has no effect on CRP; this suggests that the reduction in fibrinogen is due to an effect on its metabolism rather than suppression of an inflammatory response.
Figures

References
-
- Resch KL, Ernst E, Matrai A, Paulsen HF. Fibrinogen and viscosity as risk factors for subsequent cardiovascular events in stroke survivors. Ann Intern Med. 1992;117:371–375. - PubMed
-
- Banerjee AK, Pearson J, Gilliland EL, Goss D, Lewis JD, Stirling Y, Meade TW. A six year prospective study of fibrinogen and other risk factors associated with mortality in stable claudicants. Thromb Haemost. 1992;68:261–263. - PubMed
-
- Koster T, Rosendaal FR, Reitsma PH, van der Velden PA, Briet E, Vandenbroucke JP. Factor VII and fibrinogen levels as risk factors for venous thrombosis. Thromb Haemost. 1994;71:719–722. - PubMed
-
- Montalescot G, Ankri A, Vicaut E, Drobinnski G, Grosgogeat Y, Thomas D. Fibrinogen after coronary angioplasty as a risk factor for restenosis. Circulation. 1995;92:31–38. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

