Cyclin A transcriptional suppression is the major mechanism mediating homocysteine-induced endothelial cell growth inhibition
- PMID: 11806997
- PMCID: PMC5539868
Cyclin A transcriptional suppression is the major mechanism mediating homocysteine-induced endothelial cell growth inhibition
Abstract
Previously, it was reported that homocysteine (Hcy) specifically inhibits the growth of endothelial cells (ECs), suppresses Ras/mitogen-activated protein (MAP) signaling, and arrests cell growth at the G(1)/S transition of the cell cycle. The present study investigated the molecular mechanisms underlying this cell-cycle effect. Results showed that clinically relevant concentrations (50 microM) of Hcy significantly inhibited the expression of cyclin A messenger RNA (mRNA) in ECs in a dose- and time-dependent manner. G(1)/S-associated molecules that might account for this block were not changed, because Hcy did not affect mRNA and protein expression of cyclin D1 and cyclin E. Cyclin D1- and E-associated kinase activities were unchanged. In contrast, cyclin A-associated kinase activity and CDK2 kinase activity were markedly suppressed. Nuclear run-on assay demonstrated that Hcy decreased the transcription rate of the cyclin A gene but had no effect on the half-life of cyclin A mRNA. In transient transfection experiments, Hcy significantly inhibited cyclin A promoter activity in endothelial cells, but not in vascular smooth muscle cells. Finally, adenovirus-transduced cyclin A expression restored EC growth inhibition and overcame the S phase block imposed by Hcy. Taken together, these findings indicate that cyclin A is a critical functional target of Hcy-mediated EC growth inhibition.
Figures

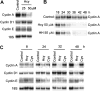


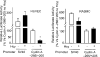


Similar articles
-
Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin A gene.Blood. 2007 Nov 15;110(10):3648-55. doi: 10.1182/blood-2007-06-096701. Epub 2007 Aug 13. Blood. 2007. PMID: 17698632 Free PMC article.
-
Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis.Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6369-73. doi: 10.1073/pnas.91.14.6369. Proc Natl Acad Sci U S A. 1994. PMID: 8022789 Free PMC article.
-
Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine.J Biol Chem. 1997 Oct 3;272(40):25380-5. doi: 10.1074/jbc.272.40.25380. J Biol Chem. 1997. PMID: 9312159
-
Regulation of cdk2 activity in endothelial cells that are inhibited from growth by cell contact.Arterioscler Thromb Vasc Biol. 2000 Mar;20(3):629-35. doi: 10.1161/01.atv.20.3.629. Arterioscler Thromb Vasc Biol. 2000. PMID: 10712384
-
Hyperhomocysteinemia, DNA methylation and vascular disease.Clin Chem Lab Med. 2007;45(12):1660-6. doi: 10.1515/CCLM.2007.350. Clin Chem Lab Med. 2007. PMID: 18067449 Review.
Cited by
-
Prolonged cyclic strain inhibits human endothelial cell growth.Front Biosci (Elite Ed). 2016 Jan 1;8(1):205-12. doi: 10.2741/E761. Front Biosci (Elite Ed). 2016. PMID: 26709656 Free PMC article.
-
Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation.Arterioscler Thromb Vasc Biol. 2005 Dec;25(12):2515-21. doi: 10.1161/01.ATV.0000189559.87328.e4. Epub 2005 Oct 6. Arterioscler Thromb Vasc Biol. 2005. PMID: 16210565 Free PMC article.
-
Notoginsenoside Fc Accelerates Reendothelialization following Vascular Injury in Diabetic Rats by Promoting Endothelial Cell Autophagy.J Diabetes Res. 2019 Sep 3;2019:9696521. doi: 10.1155/2019/9696521. eCollection 2019. J Diabetes Res. 2019. PMID: 31565658 Free PMC article.
-
Hypermethylation: Causes and Consequences in Skeletal Muscle Myopathy.J Cell Biochem. 2017 Aug;118(8):2108-2117. doi: 10.1002/jcb.25841. Epub 2017 Apr 21. J Cell Biochem. 2017. PMID: 27982479 Free PMC article. Review.
-
VASCULAR INFLAMMATION AND ATHEROGENESIS ARE ACTIVATED VIA RECEPTORS FOR PAMPs AND SUPPRESSED BY REGULATORY T CELLS.Drug Discov Today Ther Strateg. 2008;5(2):125-142. doi: 10.1016/j.ddstr.2008.11.003. Drug Discov Today Ther Strateg. 2008. PMID: 19578482 Free PMC article.
References
-
- Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–881. - PubMed
-
- Malinow MR. Hyperhomocyst(e)inemia: a common and easily reversible risk factor for occlusive atherosclerosis [published erratum appears in Circulation. 1990;82:1547] Circulation. 1990;81:2004–2006. - PubMed
-
- Verhoef P, Meleady R, Daly LE, et al. Homocysteine, vitamin status and risk of vascular disease: effects of gender and menopausal status. European COMAC Group [see comments] Eur Heart J. 1999;20:1234–1244. - PubMed
-
- Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project [see comments] JAMA. 1997;277:1775–1781. - PubMed
-
- Verhoef P, Kok FJ, Kruyssen DA, et al. Plasma total homocysteine, B vitamins, and risk of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:989–995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
